As filed with the Securities and Exchange Commission on October 27, 2022
PART II - INFORMATION REQUIRED IN OFFERING CIRCULAR
Preliminary Offering Circular dated October 27, 2022
An offering statement pursuant to Regulation A relating to these securities has been filed with the United States Securities and Exchange Commission (the “SEC”). Information contained in this Preliminary Offering Circular is subject to completion or amendment. These securities may not be sold nor may offers to buy be accepted before the offering statement filed with the SEC is qualified. This Preliminary Offering Circular shall not constitute an offer to sell or the solicitation of an offer to buy nor may there be any sales of these securities in any state in which such offer, solicitation or sale would be unlawful before registration or qualification under the laws of any such state. We may elect to satisfy our obligation to deliver a Final Offering Circular by sending you a notice within two business days after the completion of our sale to you that contains the URL where the Final Offering Circular or the offering statement in which such Final Offering Circular was filed may be obtained.
OFFERING CIRCULAR
InnovaQor, Inc.
75,000,000 Shares of Common Stock
By this Offering Circular, InnovaQor, Inc., a Nevada corporation known as VisualMED Clinical Solutions Corp. in the public trading markets, is offering for sale a maximum of 75,000,000 shares of its common stock (the “Offered Shares”), at a fixed price of $____[0.005-0.01] per share, pursuant to Tier 2 of Regulation A of the United States Securities and Exchange Commission (the “SEC”). A minimum purchase of $5,000 of the Offered Shares is required in this offering; any additional purchase must be in an amount of at least $1,000. This offering is being conducted on a best-efforts basis, which means that there is no minimum number of Offered Shares that must be sold by us for this offering to close; thus, we may receive no or minimal proceeds from this offering. All proceeds from this offering will become immediately available to us and may be used as they are accepted. Purchasers of the Offered Shares will not be entitled to a refund and could lose their entire investments. Please see the “Risk Factors” section, beginning on page 5, for a discussion of the risks associated with a purchase of the Offered Shares.
We estimate that this offering will commence within two days of qualification; this offering will terminate at the earliest of (a) the date on which the maximum offering has been sold, (b) the date which is one year from this offering being qualified by the SEC or (c) the date on which this offering is earlier terminated by us, in our sole discretion. (See “Plan of Distribution”).
Title of Securities Offered | Number of Shares | Price to Public | Commissions (1) | Proceeds to Company (2) | ||||||||||||
| Common Stock | 75,000,000 | $ | [0.005-0.01] | $ | -0- | $ | [375,000-750,000] | |||||||||
| (1) | We may offer the Offered Shares through registered broker-dealers and we may pay finders. However, information as to any such broker-dealer or finder shall be disclosed in an amendment to this Offering Circular. | |
| (2) | Does not account for the payment of expenses of this offering estimated at $20,000. See “Plan of Distribution.” |
Our common stock is quoted in the over-the-counter under the symbol “VMCS” in the OTC Pink marketplace of OTC Link. On October 24, 2022, the closing price of our common stock was $0.0064 per share.
Investing in the Offered Shares is speculative and involves substantial risks, including the superior voting rights of our outstanding shares of Series A-1 Supermajority Voting Preferred Stock, which could have the effect of precluding current and future owners of our common stock, including the Offered Shares, from influencing any corporate decision. You should purchase Offered Shares only if you can afford a complete loss of your investment. See Risk Factors, beginning on page 5, for a discussion of certain risks that you should consider before purchasing any of the Offered Shares.
The Series A-1 Supermajority Voting Preferred Stock has the following voting rights: so long as one share of Series A-1 Supermajority Voting Preferred Stock is outstanding, the outstanding share(s) of the Series A-1 Supermajority Voting Preferred Stock shall have the number of votes, in the aggregate, equal to 51% of all votes entitled to be voted at any meeting of our shareholder meeting. The current owner of all outstanding shares of the Series A-1 Supermajority Voting Preferred Stock, Epizon Limited, will, therefore, be able to control the management and affairs of our company, as well as matters requiring the approval by our shareholders, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets, and any other significant corporate transaction. (See “Risk Factors—Risks Related to a Purchase of the Offered Shares” and “Security Ownership of Certain Beneficial Owners and Management”).
THE SEC DOES NOT PASS UPON THE MERITS OF, OR GIVE ITS APPROVAL TO, ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE SEC. HOWEVER, THE SEC HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED ARE EXEMPT FROM REGISTRATION.
The use of projections or forecasts in this offering is prohibited. No person is permitted to make any oral or written predictions about the benefits you will receive from an investment in Offered Shares.
No sale may be made to you in this offering, if you do not satisfy the investor suitability standards described in this Offering Circular under “Plan of Distribution—State Law Exemption and Offerings to ‘Qualified Purchasers’” (page 19). Before making any representation that you satisfy the established investor suitability standards, we encourage you to review Rule 251(d)(2)(i)(C) of Regulation A. For general information on investing, we encourage you to refer to www.investor.gov.
This Offering Circular follows the disclosure format of Form S-1, pursuant to the General Instructions of Part II(a)(1)(ii) of Form 1-A.
The date of this Offering Circular is _________, 2022.
TABLE OF CONTENTS
| 2 |
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
The information contained in this Offering Circular includes some statements that are not historical and that are considered forward-looking statements. Such forward-looking statements include, but are not limited to, statements regarding our development plans for our business; our strategies and business outlook; anticipated development of our company; and various other matters (including contingent liabilities and obligations and changes in accounting policies, standards and interpretations). These forward-looking statements express our expectations, hopes, beliefs and intentions regarding the future. In addition, without limiting the foregoing, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words anticipates, believes, continue, could, estimates, expects, intends, may, might, plans, possible, potential, predicts, projects, seeks, should, will, would and similar expressions and variations, or comparable terminology, or the negatives of any of the foregoing, may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
The forward-looking statements contained in this Offering Circular are based on current expectations and beliefs concerning future developments that are difficult to predict. We cannot guarantee future performance, or that future developments affecting our company will be as currently anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements.
All forward-looking statements attributable to us are expressly qualified in their entirety by these risks and uncertainties. These risks and uncertainties, along with others, are also described below in the Risk Factors section. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. You should not place undue reliance on any forward-looking statements and should not make an investment decision based solely on these forward-looking statements. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
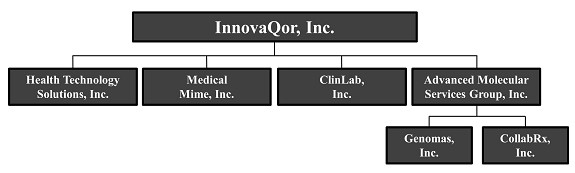
The following summary highlights material information contained in this Offering Circular. This summary does not contain all of the information you should consider before purchasing our common stock. Before making an investment decision, you should read this Offering Circular carefully, including the Risk Factors section and the consolidated financial statements and the notes thereto. Unless otherwise indicated, the terms “InnovaQor,” “the Company”, “we”, “us” and “our” refer and relate to InnovaQor, Inc., a Nevada corporation known as VisualMED Clinical Solutions Corp. in the public trading markets, including our subsidiaries: Health Technology Solutions, Inc., Medical Mime, Inc., ClinLab, Inc., Advanced Molecular Services Group, Inc., Genomas, Inc. and CollabRx, Inc.
| 3 |
Our Company
Our company, InnovaQor, Inc., a Nevada corporation, was originally incorporated in the State of Nevada on September 7, 1999, under the name Ancona Mining Corporation. On November 30, 2004, our corporate name changed to VisualMED Clinical Solutions Corporation (“VisualMED”). On September 8, 2021, our corporate name changed to InnovaQor, Inc. However, in the public trading markets, our company is still known as VisualMED Clinical Solutions Corporation. We have filed with FINRA to change our trading symbol.
The Company provides information technology solutions and services to healthcare and laboratory customers in the United States. Our goal is to develop and deliver a technology-based social media and communication platform to a broad range of healthcare professionals and businesses using a subscription revenue model with added value bolt on services. The Company, through an acquisition that closed on June 25, 2021, has a number of fully developed products and services which it offers through six wholly-owned subsidiaries that provide medical support services primarily to clinical laboratories, corporate operations, rural hospitals, physician practices and behavioral health/substance abuse centers.
Each of the subsidiaries is wholly owned by the Company and complements each other, allowing for cross selling of products and services. The Company believes the current solutions will become an added value option to a technology-based social media communication platform to a broad range of healthcare professionals and businesses using a subscription revenue model with added value bolt on services the Company plans to develop.
In the coming year we plan to develop, acquire or license and offer a telehealth solution through corporate partnerships in the emerging health technology sector. (See “Business”).
Offering Summary
| Securities Offered | The Offered Shares, 75,000,000 shares of common stock, are being offered by our company. | |
| Offering Price Per Share | $____[0.005-0.01] per Offered Share. | |
Shares Outstanding Before This Offering |
234,953,286 shares of common stock issued and outstanding as of the date of this Offering Circular. | |
Shares Outstanding After This Offering |
309,953,286 shares of common stock issued and outstanding, assuming a maximum offering hereunder. | |
Minimum Number of Shares to Be Sold in This Offering |
None | |
| Disparate Voting Rights | Our outstanding shares of Series A-1 Supermajority Voting Preferred Stock possess superior voting rights, which effectively preclude current and future owners of our common stock, including the Offered Shares, from influencing any corporate decision. The Series A-1 Supermajority Voting Preferred Stock has the following voting rights: so long as one share of Series A-1 Supermajority Voting Preferred Stock is outstanding, the outstanding share(s) of the Series A-1 Supermajority Voting Preferred Stock shall have the number of votes, in the aggregate, equal to 51% of all votes entitled to be voted at any meeting of our shareholder meeting. Epizon Limited (see Note 5 to the ownership table under “Security Ownership of Certain Beneficial Owners and Management” for information concerning beneficial ownership and control of Epizon Limited), as the current owner of all outstanding shares of the Series A-1 Supermajority Voting Preferred Stock, will, therefore, be able to control the management and affairs of our company, as well as matters requiring the approval by our shareholders, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets, and any other significant corporate transaction. (See “Risk Factors—Risks Related to a Purchase of the Offered Shares”). | |
| Investor Suitability Standards | The Offered Shares are being offered and sold only to “qualified purchasers” (as defined in Regulation A under the Securities Act). “Qualified purchasers” include: (a) “accredited investors” under Rule 501(a) of Regulation D and (b) all other investors so long as their investment in the Offered Shares does not represent more than 10% of the greater of their annual income or net worth (for natural persons), or 10% of the greater of annual revenue or net assets at fiscal year-end (for non-natural persons). | |
| Market for our Common Stock | Our common stock is quoted in the over-the-counter market under the symbol “VMCS” in the OTC Pink marketplace of OTC Link. | |
| Termination of this Offering | This offering will terminate at the earliest of (a) the date on which the maximum offering has been sold, (b) the date which is one year from this offering being qualified by the SEC and (c) the date on which this offering is earlier terminated by us, in our sole discretion. (See “Plan of Distribution”). |
| 4 |
| Use of Proceeds | We will apply the proceeds of this offering for current liabilities, existing product updates, sales and marketing, ongoing operations and new platform development. (See “Use of Proceeds”). | |
| Risk Factors | An investment in the Offered Shares involves a high degree of risk and should not be purchased by investors who cannot afford the loss of their entire investments. You should carefully consider the information included in the Risk Factors section of this Offering Circular, as well as the other information contained in this Offering Circular, prior to making an investment decision regarding the Offered Shares. | |
| Corporate Information | Our principal executive offices are located at 400 South Australian Avenue, Suite 800, West Palm Beach, Florida 33401; our telephone number is (561) 421-1900; our corporate website is located at www.innovaqor.com. No information found on our company’s website is part of this Offering Circular. |
Continuing Reporting Requirements Under Regulation A
We are required to file periodic and other reports with the SEC, pursuant to the requirements of Section 13(a) of the Securities Exchange Act of 1934. Our continuing reporting obligations under Regulation A are deemed to be satisfied, as long as we comply with our Section 13(a) reporting requirements. As a Tier 2 issuer under Regulation A, we will be required to file with the SEC a Form 1-Z (Exit Report Under Regulation A) upon the termination of this offering.
An investment in the Offered Shares involves substantial risks. You should carefully consider the following risk factors, in addition to the other information contained in this Offering Circular before purchasing any of the Offered Shares. The occurrence of any of the following risks might cause you to lose a significant part of your investment. The risks and uncertainties discussed below are not the only ones we face, but do represent those risks and uncertainties that we believe are most significant to our business, operating results, prospects and financial condition. Some statements in this Offering Circular, including statements in the following risk factors, constitute forward-looking statements. (See “Cautionary Statement Regarding Forward-Looking Statements”).
Going Concern Risk Factor
Although our financial statements have been prepared on a going concern basis, we have accumulated significant losses and have negative cash flows from operations that could adversely affect our ability to secure additional capital to fund our operations or limit our ability to react to changes in the economy or our industry. These or additional risks or uncertainties not presently known to us, or that we currently deem immaterial, raise substantial doubt about our ability to continue as a going concern. Under Accounting Standards Update (“ASU”) 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40) Accounting Standards Codification (“ASC 205-40”), InnovaQor has the responsibility to evaluate whether conditions and/or events raise substantial doubt about its ability to meet its future financial obligations as they become due within one year after the date that the financial statements are issued. As required by ASC 205-40, this evaluation shall initially not take into consideration the potential mitigating effects of plans that have not been fully implemented as of the date the financial statements are issued. Management has assessed InnovaQor’s ability to continue as a going concern in accordance with the requirement of ASC 205-40.
The accompanying consolidated financial statements have been prepared in accordance with U.S. GAAP and the rules and regulations of the SEC. The consolidated financial statements have been prepared using U.S. GAAP applicable to a going concern that contemplates the realization of assets and liquidation of liabilities in the normal course of business. InnovaQor has accumulated significant losses and has negative cash flows from operations. For the six months ended June 30, 2022, we incurred a net loss of $606,510 (unaudited) and, as of that date, we had an accumulated deficit of $18,616,660 (unaudited). For the years ended December 31, 2021 and 2020, we incurred a net loss of $845,843 and $606,451, respectively, and, as of such dates, we had an accumulated deficit of $18,010,150 and $-0-, respectively.
Additionally, we had net cash used in operating activities of $512,491 (unaudited) and $478,869 for the six months ended June 30, 2022, and the year ended December 31, 2021, respectively. At June 30, 2022, we had a working capital deficit of $3,451,460 (unaudited) and a shareholders’ deficit of $12,735,507 (unaudited). At December 31, 2021, we had a working capital deficit of $2,860,200 and a shareholders’ deficit of $12,128,997. Any losses in the future could cause the quoted price of our common stock to decline or have a material adverse effect on our financial condition, our ability to pay our debts as they become due, and on our cash flows.
Further, the Company’s cash position is critically deficient and critical payments are not being made in the ordinary course of business, all of which raises substantial doubt about InnovaQor’s ability to continue as a going concern. Management’s plans with respect to alleviating the adverse financial conditions that caused management to express substantial doubt about InnovaQor’s ability to continue as a going concern are discussed in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section.
| 5 |
InnovaQor has incurred substantial costs in connection with the acquisition of the Group which may include accounting, tax, legal and other professional services costs, recruiting and relocation costs associated with hiring key senior management personnel who are new to InnovaQor, tax costs and costs to separate information systems, among other costs. The cost of performing such functions is anticipated to be higher than the amounts reflected in InnovaQor’s historical financial statements, which would cause its future losses to increase. Accordingly, InnovaQor will continue to focus on increasing revenues.
There can be no assurance that InnovaQor will be able to achieve its business plan, raise any additional capital or secure the additional financing necessary to implement its current operating plan. The ability of InnovaQor to continue as a going concern is dependent upon its ability to significantly increase its revenues and eventually achieve profitable operations. The accompanying consolidated financial statements do not include any adjustments that might be necessary if InnovaQor is unable to continue as a going concern.
General Business and Industry Risks
An inability to retain our senior management team would be detrimental to the success of our business. We rely heavily on our senior management team; our ability to retain them is particularly important to our future success. Given the highly specialized nature of our services (Healthcare, IT), the senior management team must have a thorough understanding of our product and service offerings as well as the skills and experience necessary to manage an organization consisting of a diverse group of professionals and external parties. In addition, we rely on our senior management team to generate and market our business successfully in a crowded, complex and legislatively bound marketplace. Further, our senior management’s personal reputations and relationships with our clients are a critical element in obtaining and maintaining client engagements. We will enter into non-solicitation agreements with our senior management team, and we will also enter into well scoped non-competition agreements. If one or more members of our senior management team leave and we cannot replace them with a suitable candidate quickly, we could experience difficulty in securing and successfully completing engagements and managing our business properly, which could harm our business prospects and results of operations.
Our inability to hire and retain talented people in an industry where there is great competition for talent could have a serious negative effect on our prospects and results of operations. Our business involves the delivery of software products and professional services and is labor intensive. Our success depends largely on our general ability to attract, develop, motivate, and retain highly skilled professionals. Further, we must successfully maintain the right mix of professionals with relevant experience and skill sets as we grow, as we expand into new service offerings, and as the market evolves. The loss of a significant number of our professionals, the inability to attract, hire, develop, train, and retain additional skilled personnel, or failure to maintain the right mix of professionals could have a serious negative effect on us, including our ability to manage, staff, and successfully complete our existing engagements and obtain new engagements. Qualified professionals are in great demand, and we face significant competition for both senior and junior professionals with the requisite credentials and experience. Our principal competition for talent comes from other software and consulting firms as well as from organizations seeking to staff their internal professional positions. Many of these competitors may be able to offer significantly greater compensation and benefits or more attractive lifestyle choices, career paths, or geographic locations than we do. Therefore, we may not be successful in attracting and retaining the skilled persons we require to conduct and expand our operations successfully. Increasing competition for these revenue-generating professionals may also significantly increase our labor costs, which could negatively affect our margins and results of operations.
Additional hiring, departures, business acquisitions and dispositions could disrupt our operations, increase our costs or otherwise harm our business. Our business strategy is dependent in part upon our ability to grow by hiring individuals or groups of individuals and by acquiring complementary businesses. However, we may be unable to identify, hire, acquire, or successfully integrate new employees and acquired businesses without substantial expense, delay, or other operational or financial obstacles. From time to time, we will evaluate the total mix of products and services we provide and we may conclude that businesses may not achieve the results we previously expected. Competition for future hiring and acquisition opportunities in our markets could increase the compensation we offer to potential employees or the prices we pay for businesses we wish to acquire. In addition, we may be unable to achieve the financial, operational, and other benefits we anticipate from any hiring or acquisition, as well as any disposition, including those we have completed so far. New acquisitions could also negatively impact existing practices and cause current employees to depart. Hiring additional employees or acquiring businesses could also involve a number of additional risks, including:
| ● | the diversion of management’s time, attention, and resources from managing and marketing our Company; | |
| ● | the failure to retain key acquired personnel or existing personnel who may view the acquisition unfavorably; | |
| ● | the potential loss of clients of acquired businesses; | |
| ● | the need to compensate new employees while they wait for their restrictive covenants with other institutions to expire; | |
| ● | the potential need to raise significant amounts of capital to finance a transaction or the potential issuance of equity securities that could be dilutive to our existing shareholders; | |
| ● | increased costs to improve, coordinate, or integrate managerial, operational, financial, and administrative systems; | |
| ● | the potential assumption of liabilities of an acquired business; | |
| ● | the inability to attain the expected synergies with an acquired business; |
| 6 |
| ● | the usage of earn-outs based on the future performance of our business acquisitions may deter the acquired company from fully integrating into our existing business; | |
| ● | the perception of inequalities if different groups of employees are eligible for different benefits and incentives or are subject to different policies and programs; and | |
| ● | difficulties in integrating diverse backgrounds and experiences of consultants, including if we experience a transition period for newly hired consultants that results in a temporary drop in our utilization rates or margins. |
Our intangible assets primarily consist of customer relationships, trade names, customer contracts, technology and software. We evaluate our intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. No impairment charges for intangible assets were recorded in the year ended December 31, 2021, or the six months ended June 30, 2022.
Determining the fair value of a reporting unit requires us to make significant judgments, estimates, and assumptions. While we believe that the estimates and assumptions underlying our valuation methodology are reasonable, these estimates and assumptions could have a significant impact on whether or not a non-cash goodwill impairment charge is recognized and also the magnitude of any such charge. The results of an impairment analysis are as of a point in time. There is no assurance that the actual future earnings or cash flows of our reporting units will be consistent with our projections. We will monitor any changes to our assumptions and will evaluate goodwill as deemed warranted during future periods. Any significant decline in our operations could result in additional non-cash goodwill impairment charges.
Changes in capital markets, legal or regulatory requirements, and general economic or other factors beyond our control could reduce demand for our services, in which case our revenues and profitability could decline. A number of factors outside of our control affect demand for our services. These include:
| ● | fluctuations in the U.S. economy; | |
| ● | the U.S. or global financial markets and the availability, costs, and terms of credit; | |
| ● | changes in laws and regulations; and | |
| ● | other economic factors and general business conditions. |
We are not able to predict the positive or negative effects that future events or changes to the U.S. economy, financial markets, or regulatory and business environment could have on our operations.
Changes in U.S. tax laws could have a material adverse effect on our business, cash flow, results of operations and financial conditions. We are subject to income and other taxes in the U.S. at the state and federal level. Changes in applicable U.S. state or federal tax laws and regulations, or their interpretation and application, could materially affect our tax expense and profitability. The Company has not filed its federal tax returns for the last 10 years. The Company does not anticipate material adjustments of its tax liabilities when such returns are filed, but there is no guarantee that such filings will not have a material adverse effect.
Acquisition of the HTS Group will present management with new challenges that did not exist under the umbrella of its former parent. Under the former parent, management had the support of an experienced financial team, HR support and support for SEC filings. This support system does not currently exist in the current company and new challenges are presenting themselves every day. The immature knowledge and experience in these areas are likely to take longer to complete actions and will take management’s attention away from the day to day operations where it is needed to improve revenues.
If we are unable to manage fluctuations in our business successfully, we may not be able to achieve profitability. To successfully manage growth, we must periodically adjust and strengthen our operating, financial, accounting, and other systems, procedures, and controls, which could increase our costs and may adversely affect our gross profits and our ability to achieve profitability if we do not generate increased revenues to offset the costs. As a public company, our information and control systems must enable us to prepare accurate and timely financial information and other required disclosures. If we discover deficiencies in our existing information and control systems that impede our ability to satisfy our reporting requirements, we must successfully implement improvements to those systems in an efficient and timely manner.
The nature of our services and the general economic environment make it difficult to predict our future operating results. To achieve profitability, we must:
| ● | attract, integrate, retain, and motivate highly qualified professionals; | |
| ● | achieve and maintain adequate utilization and suitable billing rates for our revenue-generating professionals; | |
| ● | expand our existing relationships with our clients and identify new clients in need of our services; | |
| ● | successfully resell product/ engagements and secure new client sales/engagements every year; |
| 7 |
| ● | maintain and enhance our brand recognition; and | |
| ● | adapt quickly to meet changes in our markets, our business mix, the economic environment, the credit markets, and competitive developments. |
Our financial results could suffer if we are unable to achieve or maintain adequate utilization and suitable billing rates for our products and services. Our profitability depends to a large extent on the utilization and billing rates of our professionals. Utilization of our professionals is affected by a number of factors, including:
| ● | the number and size of client sales/ engagements; | |
| ● | the timing of the commencement, completion and termination of engagements, which in many cases is unpredictable; | |
| ● | our ability to transition our consultants efficiently from completed engagements to new engagements; | |
| ● | the hiring of additional consultants because there is generally a transition period for new consultants that results in a temporary drop in our utilization rate; | |
| ● | unanticipated changes in the scope of client engagements; | |
| ● | our ability to forecast demand for our services and thereby maintain an appropriate level of consultants; and | |
| ● | conditions affecting the industries in which we practice as well as general economic conditions. |
The billing rates of our consultants that we are able to charge are also affected by a number of factors, including:
| ● | our clients’ perception of our ability to add value through our products/services; | |
| ● | the market demand for the products/services we provide; | |
| ● | an increase in the number of sales/engagements in the government sector, which are subject to federal contracting regulations; | |
| ● | introduction of new product/services by us or our competitors; | |
| ● | our competition and the pricing policies of our competitors; and | |
| ● | current economic conditions. |
If we are unable to achieve and maintain adequate overall utilization as well as maintain or increase the billing rates for our consultants, our financial results could materially suffer. In addition, our consultants may need to perform services at the physical locations of our clients. If there are natural disasters, disruptions to travel and transportation or problems with communications systems, our ability to perform services for, and interact with, our clients at their physical locations may be negatively impacted which could have an adverse effect on our business and results of operations.
It is likely that our quarterly results of operations may fluctuate in the future as a result of certain factors, some of which may be outside of our control. A key element of our strategy is to market our products and services directly to certain specific organizations, such as health systems and hospitals, and to increase the number of our products and services utilized by existing clients. The sales cycle for some of our products and services is often lengthy and may involve significant commitment of client personnel. As a consequence, the commencement date of a client engagement often cannot be accurately forecasted. Certain of our client contracts contain terms that result in revenue that is deferred and cannot be recognized until the occurrence of certain events. As a result, the period of time between contract signing and recognition of associated revenue may be lengthy, and we are not able to predict with certainty the period in which revenue will be recognized.
Certain of our contracts provide that some portion or all of our fees are at risk if our services do not result in the achievement of certain performance targets. To the extent that any revenue is contingent upon the achievement of a performance target, we only recognize revenue upon client confirmation that the performance targets have been achieved. If a client fails to provide such confirmation in a timely manner, our ability to recognize revenue will be delayed.
Fee discounts, pressure to not increase or even decrease our rates, and less advantageous contract terms could result in the loss of clients, lower revenues and operating income, higher costs, and less profitable engagements. More discounts or write-offs than we expect in any period would have a negative impact on our results of operations.
Other fluctuations in our quarterly results of operations may be due to a number of other factors, some of which are not within our control, including:
| ● | the timing and volume of client invoices processed and payments received, which may affect the fees payable to us under certain of our engagements; | |
| ● | client decisions regarding renewal or termination of their contracts; | |
| ● | the amount and timing of costs related to the development or acquisition of technologies or businesses; and | |
| ● | unforeseen legal expenses, including litigation and other settlement gains or losses. |
| 8 |
The profitability of our fixed-fee engagements with clients may not meet our expectations if we underestimate the cost of these engagements. When making proposals for fixed-fee engagements, we estimate the costs and timing for completing the engagements. These estimates reflect our best judgment regarding the efficiencies of our methodologies and consultants as we plan to deploy them on engagements. Any increased or unexpected costs or unanticipated delays in connection with the performance of fixed-fee engagements, including delays caused by factors outside our control, could make these contracts less profitable or unprofitable, which would have an adverse effect on our profit margin.
Our business is becoming increasingly dependent on information technology and will require additional investments in order to grow and meet the demands of our clients. We depend on the use of sophisticated technologies and systems. Some of services may become dependent on the use of software applications and systems that we do not own and could become unavailable. Moreover, our technology platforms will require continuing investments by us in order to expand existing service offerings and develop complementary services. Our future success depends on our ability to adapt our services and infrastructure while continuing to improve the performance, features, and reliability of our services in response to the evolving demands of the marketplace.
Adverse changes to our relationships with key third-party vendors, or in the business of our key third-party vendors, could unfavorably impact our business. A portion of our services and solutions depends on technology or software provided by third-party vendors. Some of these third-party vendors refer potential clients to us, and others require that we obtain their permission prior to accessing their software. These third-party vendors could terminate their relationship with us without cause and with little or no notice, which could limit our service offerings and harm our financial condition and operating results. In addition, if a third-party vendor’s business changes or is reduced, that could adversely affect our business. Moreover, if third-party technology or software that is important to our business does not continue to be available or utilized within the marketplace, or if the services that we provide to clients is no longer relevant in the marketplace, our business may be unfavorably impacted.
We could experience system failures, service interruptions, or security breaches that could negatively impact our business. Our organization is comprised of employees who work on matters throughout the United States. We may be subject to disruption to our operating systems from technology events that are beyond our control, including the possibility of failures at third-party data centers, disruptions to the Internet, natural disasters, power losses, and malicious attacks. In addition, despite the implementation of security measures, our infrastructure and operating systems, including the Internet and related systems, may be vulnerable to physical break-ins, hackers, improper employee or contractor access, computer viruses, programming errors, denial-of-service attacks, or other attacks by third parties seeking to disrupt operations or misappropriate information or similar physical or electronic breaches of security. While we have taken and are taking reasonable steps to prevent and mitigate the damage of such events, including implementation of system security measures, information backup, and disaster recovery processes, those steps may not be effective and there can be no assurance that any such steps can be effective against all possible risks. We will need to continue to invest in technology in order to achieve redundancies necessary to prevent service interruptions. Access to our systems as a result of a security breach, the failure of our systems, or the loss of data could result in legal claims or proceedings, liability, or regulatory penalties and disrupt operations, which could adversely affect our business and financial results.
Our reputation could be damaged and we could incur additional liabilities if we fail to protect client and employee data through our own accord or if our information systems are breached. We rely on information technology systems to process, transmit, and store electronic information and to communicate among our locations and with our clients, partners, and employees. The breadth and complexity of this infrastructure increases the potential risk of security breaches which could lead to potential unauthorized disclosure of confidential information.
In providing services to clients, we may manage, utilize, and store sensitive or confidential client or employee data, including personal data and protected health information. As a result, we are subject to numerous laws and regulations designed to protect this information, such as the U.S. federal and state laws governing the protection of health or other personally identifiable information, including the Health Insurance Portability and Accountability Act (HIPAA). In addition, many states, and U.S. federal governmental authorities have adopted, proposed or are considering adopting or proposing, additional data security and/or data privacy statutes or regulations. Continued governmental focus on data security and privacy may lead to additional legislative and regulatory action, which could increase the complexity of doing business. The increased emphasis on information security and the requirements to comply with applicable U.S. data security and privacy laws and regulations may increase our costs of doing business and negatively impact our results of operations.
These laws and regulations are increasing in complexity and number. If any person, including any of our employees, negligently disregards or intentionally breaches our established controls with respect to client or employee data, or otherwise mismanages or misappropriates that data, we could be subject to significant monetary damages, regulatory enforcement actions, fines, and/or criminal prosecution.
In addition, unauthorized disclosure of sensitive or confidential client or employee data, whether through systems failure, employee negligence, fraud, or misappropriation, could damage our reputation and cause us to lose clients and their related revenue in the future.
| 9 |
Changes in capital markets, legal or regulatory requirements, general economic conditions and monetary or geo-political disruptions, as well as other factors beyond our control, could reduce demand for our practice offerings or services, in which case our revenues and profitability could decline. Different factors outside of our control could affect demand for our practices and our services. These include:
| ● | fluctuations in the U.S. economy, including economic recessions and the strength and rate of any general economic recoveries; | |
| ● | the U.S. financial markets and the availability, costs and terms of credit and credit modifications; | |
| ● | business and management crises, including the occurrence of alleged fraudulent or illegal activities and practices; | |
| ● | new and complex laws and regulations, repeals of existing laws and regulations or changes of enforcement of laws, rules and regulations; | |
| ● | other economic, geographic or political factors; and | |
| ● | general business conditions. |
We are not able to predict the positive or negative effects that future events or changes to the U.S. economy will have on our business. Fluctuations, changes and disruptions in financial, credit, mergers and acquisitions and other markets, political instability and general business factors could impact various segments’ operations and could affect such operations differently. Changes to factors described above, as well as other events, including by way of example, contractions of regional economies, monetary systems, banking, real estate and retail or other industries; debt or credit difficulties or defaults by businesses; new, repeals of or changes to laws and regulations, including changes to the bankruptcy and competition laws of the U.S.; tort reform; banking reform; a decline in the implementation or adoption of new laws of regulation, or in government enforcement, litigation or monetary damages or remedies that are sought; or political instability may have adverse effects on our business.
Our revenues, operating income and cash flows are likely to fluctuate. We expect to experience fluctuations in our revenues and cost structure and the resulting operating income and cash flows. We may experience fluctuations in our annual and quarterly financial results, including revenues, operating income and earnings per share, for reasons that include (i) the types and complexity, number, size, timing and duration of client engagements; (ii) the timing of revenue recognition under U.S. GAAP; (iii) the utilization of revenue-generating professionals, including the ability to adjust staffing levels up or down to accommodate our business and prospects of; (iv) the time it takes before a new hire becomes profitable; (v) the geographic locations of our clients or the locations where services are rendered; (vi) billing rates and fee arrangements, including the opportunity and ability to successfully reach milestones and complete projects, and collect for them; (vii) the length of billing and collection cycles and changes in amounts that may become uncollectible; (viii) changes in the frequency and complexity of government regulatory and enforcement activities; and (ix) economic factors beyond our control.
We may also experience fluctuations in our operating income and related cash flows because of increases in employee compensation, including changes to our incentive compensation structure and the timing of incentive payments. Also, the timing of investments or acquisitions and the cost of integrating them may cause fluctuations in our financial results, including operating income and cash flows. This volatility may make it difficult to forecast our future results with precision and to assess accurately whether increases or decreases in any one or more quarters are likely to cause annual results to exceed or fall short of expectations.
If we do not effectively manage the utilization of our professionals or billable rates, our financial results could decline. Our failure to manage the utilization of our professionals who bill on an hourly basis, or maintain or increase the hourly rates we charge our clients for our services, could result in adverse consequences, such as non- or lower-revenue-generating professionals, increased employee turnover, fixed compensation expenses in periods of declining revenues, the inability to appropriately staff engagements (including adding or reducing staff during periods of increased or decreased demand for our services), or special charges associated with reductions in staff or operations. Reductions in workforce or increases of billable rates will not necessarily lead to savings. In such events, our financial results may decline or be adversely impacted. A number of factors affect the utilization of our professionals. Some of these factors we cannot predict with certainty, including general economic and financial market conditions; the complexity, number, type, size and timing of client engagements; the level of demand for our services; appropriate professional staffing levels, in light of changing client demands and market conditions; and competition and acquisitions. In addition, any expansion into or within locations where we are not well-known or where demand for our services is not well-developed could also contribute to low or lower utilization rates.
InnovaQor may enter into engagements which involve non-time and material arrangements, such as fixed fees and time and materials with caps. Failure to effectively manage professional hours and other aspects of alternative fee engagements may result in the costs of providing such services exceeding the fees collected by InnovaQor. Failure to successfully complete or reach milestones with respect to contingent fee or success fee assignments may also lead to lower revenues or the costs of providing services under those types of arrangements may exceed the fees collected by InnovaQor.
| 10 |
We may receive requests to discount our fees or to negotiate lower rates for our services and to agree to contract terms relative to the scope of services and other terms that may limit the size of an engagement or our ability to pass through costs. We will consider these requests on a case-by-case basis. In addition, our clients and prospective clients may not accept rate increases that we put into effect or plan to implement in the future. Fee discounts, pressure not to increase or even decrease our rates, and less advantageous contract terms could result in the loss of clients, lower revenues and operating income, higher costs and less profitable engagements. More discounts or write-offs than we expect in any period would have a negative impact on our results of operations. There is no assurance that significant client engagements will be renewed or replaced in a timely manner or at all, or that they will generate the same volume of work or revenues, or be as profitable as past engagements.
Our Company faces certain risks, including (i) industry consolidation and a heightened competitive environment, (ii) downward pricing pressure, (iii) technology changes and obsolescence, (v) failure to protect client information against cyber-attacks and (vi) failure to protect IP, which individually or together could cause the financial results and prospects of the Company to decline. Our Company is facing significant competition from other consulting and/or software providers. There continues to be significant consolidation of companies providing products and services similar to those offered by our Company, which may provide competitors access to greater financial and other resources than those of InnovaQor. This industry is subject to significant and rapid innovation. Larger competitors may be able to invest more in research and development, react more quickly to new regulatory or legal requirements and other changes, or innovate more quickly and efficiently. Our Medical Mime and ClinLab software has been facing significant competition from competing software products.
The software and products of our Company are subject to rapid technological innovation. There is no assurance that we will successfully develop new versions of our Medical Mime and ClinLab software or other products. Our software may not keep pace with necessary changes and innovation. There is no assurance that new, innovative or improved software or products will be developed, compete effectively with the software and technology developed and offered by competitors, be price competitive with other companies providing similar software or products, or be accepted by our clients or the marketplace. If InnovaQor is unable to develop and offer competitive software and products or is otherwise unable to capitalize on market opportunities, the impact could adversely affect our operating margins and financial results.
Our reputation for providing secure information storage and maintaining the confidentiality of proprietary, confidential and trade secret information is critical to the success of our Company, which hosts client information as a service. We may face cyber-based attacks and attempts by hackers and similar unauthorized users to gain access to or corrupt our information technology systems. Such attacks could disrupt our business operations, cause us to incur unanticipated losses or expenses, and result in unauthorized disclosures of confidential or proprietary information. Although we seek to prevent, detect and investigate these network security incidents, and have taken steps to mitigate the likelihood of network security breaches, there can be no assurance that attacks by unauthorized users will not be attempted in the future or that our security measures will be effective.
We rely on a combination of copyrights, trademarks, trade secrets, confidentiality and other contractual provisions to protect our assets. Our software and related documentation will be protected principally under trade secret and copyright laws, which afford only limited protection, and the laws of some foreign jurisdictions provide less protection for our proprietary rights than the laws of the U.S. Unauthorized use and misuse of our IP by employees or third parties could have a material adverse effect on our business, financial condition and results of operations. The available legal remedies for unauthorized or misuse of our IP may not adequately compensate us for the damages caused by unauthorized use.
If we (i) fail to compete effectively, including by offering our software and services at a competitive price, (ii) are unable to keep pace with industry innovation and user requirements, (iii) are unable to replace clients or revenues as engagements end or are canceled or the scope of engagements are curtailed, or (iv) are unable to protect our clients’ or our own IP and proprietary information, the financial results of InnovaQor would be adversely affected. There is no assurance that we can replace clients or the revenues from engagements, eliminate the costs associated with those engagements, find other engagements to utilize our professionals, develop competitive products or services that will be accepted or preferred by users, offer our products and services at competitive prices, or continue to maintain the confidentiality of our IP and the information of our clients.
We may not manage our growth effectively, and our profitability may suffer. Periods of expansion may strain our management team, or human resources and information systems. To manage growth successfully, we may need to add qualified managers and employees and periodically update our operating, financial and other systems, as well as our internal procedures and controls. We also must effectively motivate, train and manage a larger professional staff. If we fail to add or retain qualified managers, employees and contractors when needed, estimate costs, or manage our growth effectively, our business, financial results and financial condition may suffer.
We cannot assure that we can successfully manage growth through acquisitions and the integration of the companies and assets we acquire or that they will result in the financial, operational and other benefits that we anticipate. Some acquisitions may not be immediately accretive to earnings, and some expansion may result in significant expenditures.
| 11 |
In periods of declining growth, underutilized employees and contractors may result in expenses and costs being a greater percentage of revenues. In such situations, we will have to weigh the benefits of decreasing our workforce or limiting our service offerings and saving costs against the detriment that InnovaQor could experience from losing valued professionals and their industry expertise and clients.
Our business, financial condition, results of operations and growth could be harmed by the effects of the COVID-19 pandemic. We are subject to risks related to the public health crises such as the global pandemic associated with the coronavirus (COVID-19). In December 2019, a novel strain of coronavirus, SARS-CoV-2, was reported to have surfaced in Wuhan, China. Since then, SARS-CoV-2, and the resulting disease COVID-19, has spread to most countries, and all 50 states within the United States. In March 2020, the World Health Organization declared the COVID-19 outbreak a pandemic. Further, the President of the United States declared the COVID-19 pandemic a national emergency, invoking powers under the Stafford Act, the legislation that directs federal emergency disaster response, and under the Defense Production Act, the legislation that facilitates the production of goods and services necessary for national security and for other purposes. Numerous governmental jurisdictions have imposed, and others in the future may impose, “shelter-in-place” orders, quarantines, executive orders and similar government orders and restrictions for their residents to control the spread of COVID-19. Most states and the federal government have declared a state of emergency related to the spread of COVID-19. Such orders or restrictions, and the perception that such orders or restrictions could occur, have resulted in business closures, work stoppages, slowdowns and delays, work-from-home policies, travel restrictions and cancellation of events, among other effects, thereby negatively impacting our customers, employees, and offices, among others. We may experience further limitations on employee resources in the future, because of sickness of employees or their families.
Healthcare organizations around the world, including our health care provider customers, have faced and will continue to face, substantial challenges in treating patients with COVID-19, such as the diversion of staff and resources from ordinary functions to the treatment of COVID-19, supply, resource and capital shortages and overburdening of staff and resource capacity. In the United States, governmental authorities have also recommended, and in certain cases required, that elective, specialty and other procedures and appointments, including certain primary care services, be suspended or canceled to avoid non-essential patient exposure to medical environments and potential infection with COVID-19 and to focus limited resources and personnel capacity toward the treatment of COVID-19. These measures and challenges will likely continue for the duration of the pandemic, which is uncertain, and will disproportionately harm the results of operations, liquidity and financial condition of these health care organizations and our health care provider customers. As a result, our health care provider customers may seek contractual accommodations from us in the future. To the extent such health care provider customers experience challenges and difficulties, it will adversely affect our business operation and results of operations. Further, a recession or prolonged economic contraction as a result of the COVID-19 pandemic could also harm the business and results of operations of our customers, resulting in potential business closures, layoffs of employees and a significant increase in unemployment in the United States which may continue even after the pandemic. The occurrence of any such events may lead to reduced income for customers and reduced size of workforces, which could reduce our revenue and harm our business, financial condition and results of operations.
The widespread COVID-19 pandemic has resulted in, and may continue to result in, significant volatility and uncertainty in U.S. financial markets, which may reduce our ability to access capital, which could in the future negatively affect our liquidity. In addition, a recession or market correction resulting from the spread of COVID-19 could materially affect our business and the value of our common stock.
Further, given the dislocation and government-imposed travel related limitations as a consequence of the COVID-19 pandemic, our ability to complete acquisitions in the near-term may be delayed. Future acquisitions may be subject to difficulties in evaluating potential acquisition targets as a result of the inability to accurately predict the duration or long-term economic and business consequences resulting from the COVID-19 pandemic.
The global outbreak of COVID-19 continues to rapidly evolve. We have taken steps intended to mitigate the effects of the pandemic and to protect our workforce. Although we believe we have taken the appropriate actions, we cannot guarantee that these measures will mitigate all or any negative effects of the pandemic. The ultimate impact of the COVID-19 pandemic or a similar health epidemic is highly uncertain and subject to change. We cannot at this time precisely predict what effects the COVID-19 outbreak will have on our business, results of operations and financial condition, including the uncertainties relating to the ultimate geographic spread of the virus, the severity of the disease, the duration of the pandemic, the availability of vaccinations, and the governmental responses to the pandemic. However, we will continue to monitor the COVID-19 situation closely and are committed to continuing to make appropriate changes as and when needed.
| 12 |
We currently do not have sufficient cash to fully implement our business plan. We have experienced a lack of adequate capital resources causing us to be unable to fully implement our full business plan. We believe that we need to raise or otherwise obtain additional financing beyond our current cash position in order to satisfy our existing obligations and fully implement our business plan. We do not expect to have positive cash flow until the end of 2023 or longer. If we are not successful in obtaining additional financing, including pursuant to this offering, we will not be able to fully implement our business plan and we may not be able to continue our operations.
We may not secure the capital required to develop our business. Our business is dependent on securing additional capital. If we fail to secure the required capital, including in this offering, our business will fail.
Our business plan is not based on independent market studies. We have not commissioned any independent market studies concerning our business plans. Rather, our plans for implementing our business strategy and achieving profitability are based on the experience, judgment and assumptions of our management. If these assumptions prove to be incorrect, we may not be successful in our business operations.
Our Board of Directors may change our policies without shareholder approval. Our policies, including any policies with respect to investments, leverage, financing, growth, debt and capitalization, will be determined by our Board of Directors or officers to whom our Board of Directors delegate such authority. Our Board of Directors will also establish the amount of any dividends or other distributions that we may pay to our shareholders. Our Board of Directors or officers to which such decisions are delegated will have the ability to amend or revise these and our other policies at any time without shareholder vote. Accordingly, our shareholders will not be entitled to approve changes in our policies, which policy changes may have a material adverse effect on our financial condition and results of operations.
Risks Related to Our Organization and Structure
Our holding company structure makes us dependent on our subsidiaries for our cash flow and could serve to subordinate the rights of our shareholders to the rights of creditors of our subsidiaries, in the event of an insolvency or liquidation of any such subsidiary. Our company acts as a holding company and, accordingly, substantially all of our operations are conducted through our subsidiaries. Such subsidiaries will be separate and distinct legal entities. As a result, substantially all of our cash flow will depend upon the earnings of our subsidiaries. In addition, we will depend on the distribution of earnings, loans or other payments by our subsidiaries. No subsidiary will have any obligation to provide our company with funds for our payment obligations. If there is an insolvency, liquidation or other reorganization of any of our subsidiaries, our shareholders will have no right to proceed against their assets. Creditors of those subsidiaries will be entitled to payment in full from the sale or other disposal of the assets of those subsidiaries before our company, as a shareholder, would be entitled to receive any distribution from that sale or disposal.
Risks Related to a Purchase of the Offered Shares
The outstanding shares of our Series A-1 Supermajority Voting Preferred Stock preclude current and future owners of our common stock from influencing any corporate decision. Epizon Limited (see Note 5 to the ownership table under “Security Ownership of Certain Beneficial Owners and Management” for information concerning beneficial ownership and control of Epizon Limited) owns all of the outstanding shares of our Series A-1 Supermajority Voting Preferred Stock. The Series A-1 Supermajority Voting Preferred Stock has the following voting rights: so long as one share of Series A-1 Supermajority Voting Preferred Stock is outstanding, the outstanding share(s) of the Series A-1 Supermajority Voting Preferred Stock shall have the number of votes, in the aggregate, equal to 51% of all votes entitled to be voted at any meeting of our shareholder meeting. Epizon Limited will, therefore, be able to control the management and affairs of our company, as well as matters requiring the approval by our shareholders, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets, and any other significant corporate transaction. (See “Security Ownership of Certain Beneficial Owners and Management”).
We may seek capital that may result in shareholder dilution or that may have rights senior to those of our common stock, including the Offered Shares. From time to time, we may seek to obtain additional capital, either through equity, equity-linked or debt securities. The decision to obtain additional capital will depend on, among other factors, our business plans, operating performance and condition of the capital markets. If we raise additional funds through the issuance of equity, equity-linked or debt securities, those securities may have rights, preferences or privileges senior to the rights of our common stock, which could negatively affect the market price of our common stock or cause our shareholders to experience dilution.
We do not intend to pay dividends on our common stock. We intend to retain earnings, if any, to provide funds for the implementation of our business strategy. We do not intend to declare or pay any dividends in the foreseeable future. Therefore, there can be no assurance that holders of our common stock will receive cash, stock or other dividends on their shares of our common stock, until we have funds which our Board of Directors determines can be allocated to dividends.
| 13 |
Our common stock has been, and may in the future be, a “Penny Stock” and subject to specific rules governing its sale to investors. The SEC has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to our Common Stock, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require that a broker or dealer approve a person’s account for transactions in penny stocks; and the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must obtain financial information and investment experience objectives of the person; and make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth the basis on which the broker or dealer made the suitability determination; and that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors sell shares of our common stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
There is minimal trading activity in our common stock and there is no assurance that an active market will develop in the future. Although our common stock is currently quoted on the OTC Pink marketplace of OTC Link (an interdealer electronic quotation system operated by OTC Markets Group, Inc.) under the symbol “VMCS”, trading of our common stock may be extremely sporadic. For example, several days may pass before any shares may be traded. As a result, an investor may find it difficult to dispose of, or to obtain accurate quotations of the price of our common stock. There can be no assurance that a more active market for our common stock will develop, or if one should develop, there is no assurance that it will be sustained. This severely limits the liquidity of our common stock, and would likely have a material adverse effect on the market price of our common stock and on our ability to raise additional capital.
The market for our common stock may be volatile; you could lose all or part of your investment in the Offered Shares. The market price of our common stock may fluctuate substantially and will depend on a number of factors many of which are beyond our control and may not be related to our operating performance. These fluctuations could cause you to lose all or part of your investment in the Offered Shares, since you might be unable to sell your Offered Shares at or above the price you pay for the Offered Shares. Factors that could cause fluctuations in the market price of our common stock include, but are not limited to, the following:
| ● | price and volume fluctuations in the overall stock market from time to time; | |
| ● | volatility in the market prices and trading volumes of small-company stocks; | |
| ● | changes in operating performance and stock market valuations of other similar companies generally; | |
| ● | sales of shares of our common stock by us or our shareholders; | |
| ● | failure of securities analysts to maintain coverage of us, changes in financial estimates by securities analysts who follow our company, or our failure to meet these estimates or the expectations of investors; | |
| ● | the financial projections we may provide to the public, any changes in those projections or our failure to meet those projections; | |
| ● | announcements by us or our competitors of new products or services; | |
| ● | the public’s reaction to our press releases, other public announcements and filings with the SEC; | |
| ● | rumors and market speculation involving us or other companies in our industry; | |
| ● | actual or anticipated changes in our operating results or fluctuations in our operating results; | |
| ● | actual or anticipated developments in our business, our competitors’ businesses or the competitive landscape generally; | |
| ● | litigation involving us, our industry or both, or investigations by regulators into our operations or those of our competitors; | |
| ● | developments or disputes concerning our intellectual property or other proprietary rights; | |
| ● | announced or completed acquisitions of businesses or technologies by us or our competitors; | |
| ● | new laws or regulations or new interpretations of existing laws or regulations applicable to our business; | |
| ● | changes in accounting standards, policies, guidelines, interpretations or principles; | |
| ● | any significant change in our management; and | |
| ● | general economic conditions and slow or negative growth of our markets. |
| 14 |
In addition, in the past, following periods of volatility in the overall market and the market price of a particular company’s securities, securities class action litigation has often been instituted against these companies. This litigation, if instituted against us, could result in substantial costs and a diversion of our management’s attention and resources.
Compliance with the reporting requirements of federal securities laws can be expensive. We are a public reporting company in the United States, and accordingly, subject to the information and reporting requirements of the Exchange Act and other federal securities laws, and the compliance obligations of the Sarbanes-Oxley Act. The costs of preparing and filing annual and quarterly reports and other required information with the SEC, furnishing audited reports to shareholders and preparing any registration statements from time to time, if any, are substantial.
Applicable regulatory requirements, including those contained in and issued under the Sarbanes-Oxley Act of 2002, may make it difficult for us to retain or attract qualified officers and directors, which could adversely affect the management of our business and our ability to obtain or retain listing of our common stock. We may be unable to attract and retain those qualified officers, directors and members of board committees required to provide for effective management because of the rules and regulations that govern publicly held companies, including, but not limited to, certifications by principal executive officers. The enactment of the Sarbanes-Oxley Act has resulted in the issuance of a series of related rules and regulations and the strengthening of existing rules and regulations by the SEC, as well as the adoption of new and more stringent rules by the stock exchanges. The perceived increased personal risk associated with these changes may deter qualified individuals from accepting roles as directors and executive officers.
Further, some of these changes heighten the requirements for board or committee membership, particularly with respect to an individual’s independence from the corporation and level of experience in finance and accounting matters. While certain board and committee requirements may not apply to us as an OTC listed company, we intend to explore voluntarily complying with some of these requirements. We may have difficulty attracting and retaining directors with the requisite qualifications. If we are unable to attract and retain qualified officers and directors, the management of our business and our ability to obtain or retain listing of our shares of common stock on any stock exchange (assuming we elect to seek and are successful in obtaining such listing) could be adversely affected.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or detect fraud, and, consequently, investors could lose confidence in our financial reporting and this may decrease the trading price of our common stock. We must maintain effective internal controls to provide reliable financial reports and detect fraud. We have been assessing our internal controls to identify areas that need improvement. We are in the process of implementing changes to internal controls, but have not yet completed implementing these changes. Failure to implement these changes to our internal controls or any others that it identifies as necessary to maintain an effective system of internal controls could harm our operating results and cause investors to lose confidence in our reported financial information. Any such loss of confidence would have a negative effect on the trading price of our stock.
Our Articles of Incorporation allows for our board to create new series of preferred stock without further approval by our shareholders, which could adversely affect the rights of the holders of our common stock, including purchasers of the Offered Shares. Our board of directors has the authority to issue shares of our preferred stock, with such relative rights and preferences as the board of directors may determine, without further shareholder approval. As a result, our board of directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation and the right to receive dividend payments before dividends are distributed to the holders of our common stock. In addition, our board of directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing shareholders.
You will suffer dilution in the net tangible book value of the Offered Shares you purchase in this offering. If you acquire any Offered Shares, you will suffer immediate dilution, due to the lower book value per share of our common stock compared to the purchase price of the Offered Shares in this offering. (See “Dilution”).
Future issuances of debt securities and equity securities could negatively affect the market price of shares of our common stock and, in the case of equity securities, may be dilutive to existing shareholders. In the future, we may issue debt or equity securities or incur other financial obligations, including stock dividends. Upon liquidation, it is possible that holders of our debt securities and other loans and preferred stock would receive a distribution of our available assets before common shareholders. We are not required to offer any such additional debt or equity securities to existing shareholders on a preemptive basis. Therefore, additional common stock issuances, directly or through convertible or exchangeable securities, warrants or options, would dilute the holdings of our existing common shareholders and such issuances, or the perception of such issuances, could reduce the market price of shares of our common stock.
As an issuer of penny stock, the protection provided by the federal securities laws relating to forward-looking statements does not apply to us. Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to issuers of penny stocks. As a result, we will not have the benefit of this safe harbor protection, in the event of any legal action based upon a claim that the material provided by us contained a material misstatement of fact or was misleading in any material respect because of our failure to include any statements necessary to make the statements not misleading. Such an action could hurt our financial condition.
| 15 |
Ownership Dilution
The information under “Investment Dilution” below does not take into account the potential conversion of the outstanding shares of (a) Series B-1 Convertible Redeemable Preferred Stock into a total of approximately 3,800,000,000 shares of our common stock and (b) Series C-1 Convertible Redeemable Preferred Stock into a total of approximately 55,000,000 shares of our common stock, based on recent market prices of our common stock. While it is unlikely that any such numbers of shares would be issued in a short period of time, due to a 4.99% “equity blocker” provision, it is important for holders of our common stock, including the Offered Shares, to note that they can expect to incur significant dilution in their ownership of our company. (See “Risk Factors—Risks Related to a Purchase of the Offered Shares,” “Description of Securities—Preferred Stock—Series A-1 Supermajority Voting Preferred Stock,” and “Security Ownership of Certain Beneficial Owners and Management”).
Investment Dilution
Dilution in net tangible book value per share to purchasers of our common stock in this offering represents the difference between the amount per share paid by purchasers of the Offered Shares in this offering and the pro forma as adjusted net tangible book value per share immediately after completion of this offering. In this offering, dilution is attributable primarily to our relatively low net tangible book value per share.
If you purchase Offered Shares in this offering, your investment will be diluted to the extent of the difference between your purchase price per Offered Share and the net pro forma as adjusted tangible book value per share of our common stock after this offering. Our net tangible book value as of June 30, 2022, was $(12,735,507) (unaudited), or $(0.054) (unaudited) per share.
Without taking into account issuances of our common stock occurring after June 30, 2022, the tables below illustrate the dilution to purchasers of Offered Shares in this offering, on a pro forma basis, assuming 100%, 75%, 50% and 25% of the Offered Shares are sold.
| Assuming the Sale of 100% of the Offered Shares | ||||
| Offering price per share | $ | [0.005-0.01 | ] | |
| Net tangible book value per share as of June 30, 2022 | $ | (0.054 | ) | |
| Increase in net tangible book value per share after giving effect to this offering | $ | [0.014-0.015 | ] | |
| Pro forma net tangible book value per share as of June 30, 2022 | $ | [(0.040-0.039 | )] | |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | $ | [0.045-0.049 | ] | |
| Assuming the Sale of 75% of the Offered Shares | ||||
| Offering price per share | $ | [0.005-0.01 | ] | |
| Net tangible book value per share as of June 30, 2022 | $ | (0.054 | ) | |
| Increase in net tangible book value per share after giving effect to this offering | $ | [0.011-0.012 | ] | |
| Pro forma net tangible book value per share as of June 30, 2022 | $ | [(0.043-0.042 | )] | |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | $ | [0.048-0.052 | ] | |
| Assuming the Sale of 50% of the Offered Shares | ||||
| Offering price per share | $ | [0.005-0.01 | ] | |
| Net tangible book value per share as of June 30, 2022 | $ | (0.054 | ) | |
| Increase in net tangible book value per share after giving effect to this offering | $ | [0.008-0.009 | ] | |
| Pro forma net tangible book value per share as of June 30, 2022 | $ | [(0.046-0.045 | )] | |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | $ | [0.051-0.055 | ] | |
| Assuming the Sale of 25% of the Offered Shares | ||||
| Offering price per share | $ | [0.005-0.01 | ] | |
| Net tangible book value per share as of June 30, 2022 | $ | (0.054 | ) | |
| Increase in net tangible book value per share after giving effect to this offering | $ | [0.004-0.005 | ] | |
| Pro forma net tangible book value per share as of June 30, 2022 | $ | [(0.050-0.049 | )] | |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | $ | [0.055-0.059 | ] | |
| 16 |
The table below sets forth the estimated proceeds we would derive from this offering, assuming the sale of 25%, 50%, 75% and 100% of the Offered Shares and assuming the payment of no sales commissions or finder’s fees. There is, of course, no guaranty that we will be successful in selling any of the Offered Shares in this offering.
| Assumed Percentage of Offered Shares Sold in This Offering | ||||||||||||||||
| 25% | 50% | 75% | 100% | |||||||||||||
| Offered Shares sold | 18,750,000 | 37,500,000 | 56,250,000 | 75,000,000 | ||||||||||||
| Gross proceeds | $ | [93,750-187,500 | ] | $ | [187,500-375,000 | ] | $ | [281,250-562,500 | ] | $ | [375,000-750,000 | ] | ||||
| Offering expenses | 20,000 | 20,000 | 20,000 | 20,000 | ||||||||||||
| Net proceeds | $ | [73,750-167,500 | ] | $ | [167,500-355,000 | ] | $ | [261,250-542,500 | ] | $ | [355,000-730,000 | ] | ||||
The table below sets forth the manner in which we intend to apply the net proceeds derived by us in this offering, assuming the sale of 25%, 50%, 75% and 100% of the Offered Shares. All amounts set forth below are estimates.
Use of Proceeds for Assumed Percentage of Offered Shares Sold in This Offering | ||||||||||||||||
| 25% | 50% | 75% | 100% | |||||||||||||
| Current Liabilities | $ | [12,540-28,475 | ] | $ | [28,475-60,350 | ] | $ | [44,410-92,225 | ] | $ | [60,350-124,100 | ] | ||||
| Existing Product Updates | [5,900-13,400 | ] | [13,400-28,400 | ] | [20,900-43,400 | ] | [28,400-58,400 | ] | ||||||||
| Sales and Marketing | [5,900-13,400 | ] | [13,400-28,400 | ] | [20,900-43,400 | ] | [28,400-58,400 | ] | ||||||||
| Ongoing Operations | [12,540-28,475 | ] | [28,475-60,350 | ] | [44,410-92,225 | ] | [60,350-124,100 | ] | ||||||||
| New Platform Development | [36,870-83,750 | ] | [83,750-177,500 | ] | [130,630-271,250 | ] | [177,500-365,000 | ] | ||||||||
| TOTAL | $ | [73,750-167,500 | ] | $ | [167,500-355,000 | ] | $ | [261,250-542,500 | ] | $ | [355,000-730,000 | ] | ||||
We reserve the right to change the foregoing use of proceeds, should our management believe it to be in the best interest of our company. The allocations of the proceeds of this offering presented above constitute the current estimates of our management and are based on our current plans, assumptions made with respect to the industry in which we currently or, in the future, expect to operate, general economic conditions and our future revenue and expenditure estimates.
Investors are cautioned that expenditures may vary substantially from the estimates presented above. Investors must rely on the judgment of our management, who will have broad discretion regarding the application of the proceeds of this offering. The amounts and timing of our actual expenditures will depend upon numerous factors, including market conditions, cash generated by our operations (if any), business developments and the rate of our growth. We may find it necessary or advisable to use portions of the proceeds of this offering for other purposes.
In the event we do not obtain the entire offering amount hereunder, we may attempt to obtain additional funds through private offerings of our securities or by borrowing funds. Currently, we do not have any committed sources of financing.
| 17 |
In General
Our company is offering a maximum of 75,000,000 Offered Shares on a best-efforts basis, at a fixed price of $____[0.005-0.01] per Offered Share; any funds derived from this offering will be immediately available to us for our use. There will be no refunds. This offering will terminate at the earliest of (a) the date on which the maximum offering has been sold, (b) the date which is one year from this offering being qualified by the SEC or (c) the date on which this offering is earlier terminated by us, in our sole discretion.
There is no minimum number of Offered Shares that we are required to sell in this offering. All funds derived by us from this offering will be immediately available for use by us, in accordance with the uses set forth in the Use of Proceeds section of this Offering Circular. No funds will be placed in an escrow account during the offering period and no funds will be returned, once an investor’s subscription agreement has been accepted by us.
We intend to sell the Offered Shares in this offering through the efforts of our President and Chief Executive Officer, Sharon Hollis. Ms. Hollis will not receive any compensation for offering or selling the Offered Shares. We believe that Ms. Hollis is exempt from registration as a broker-dealer under the provisions of Rule 3a4-1 promulgated under the Securities Exchange Act of 1934 (the Exchange Act). In particular, Ms. Hollis:
| ● | is not subject to a statutory disqualification, as that term is defined in Section 3(a)(39) of the Securities Act; and | |
| ● | is not to be compensated in connection with his participation by the payment of commissions or other remuneration based either directly or indirectly on transactions in securities; and | |
| ● | is not an associated person of a broker or dealer; and | |
| ● | meets the conditions of the following: |
● primarily performs, and will perform at the end of this offering, substantial duties for us or on our behalf otherwise than in connection with transactions in securities; and
● was not a broker or dealer, or an associated person of a broker or dealer, within the preceding 12 months; and
● did not participate in selling an offering of securities for any issuer more than once every 12 months other than in reliance on paragraphs (a)(4)(i) or (iii) of Rule 3a4-1 under the Exchange Act.
As of the date of this Offering Circular, we have not entered into any agreements with selling agents for the sale of the Offered Shares. However, we reserve the right to engage FINRA-member broker-dealers. In the event we engage FINRA-member broker-dealers, we expect to pay sales commissions of up to 8.0% of the gross offering proceeds from their sales of the Offered Shares. In connection with our appointment of a selling broker-dealer, we intend to enter into a standard selling agent agreement with the broker-dealer pursuant to which the broker-dealer would act as our non-exclusive sales agent in consideration of our payment of commissions of up to 8% on the sale of Offered Shares effected by the broker-dealer.
Procedures for Subscribing
If you are interested in subscribing for Offered Shares in this offering, please submit a request for information by e-mail to Ms. Hollis, at: shollis@InnovaQor.com; all relevant information will be delivered to you by return e-mail. Thereafter, should you decide to subscribe for Offered Shares, you are required to follow the procedures described in the subscription agreement included in the delivered information, which are:
| - | Electronically execute and deliver to us a subscription agreement; and | |
| - | Deliver funds directly by check or by wire or electronic funds transfer via ACH to our specified bank account. |
Right to Reject Subscriptions
After we receive your complete, executed subscription agreement and the funds required under the subscription agreement have been transferred to us, we have the right to review and accept or reject your subscription in whole or in part, for any reason or for no reason. We will return all monies from rejected subscriptions immediately to you, without interest or deduction.
Acceptance of Subscriptions
Conditioned upon our acceptance of a subscription agreement, we will countersign the subscription agreement and issue the Offered Shares subscribed. Once you submit the subscription agreement and it is accepted, you may not revoke or change your subscription or request your subscription funds. All accepted subscription agreements are irrevocable.
| 18 |
This Offering Circular will be furnished to prospective investors upon their request via electronic PDF format and will be available for viewing and download 24 hours per day, 7 days per week on our company’s page on the SEC’s website: www.sec.gov.
An investor will become a shareholder of our company and the Offered Shares will be issued, as of the date of settlement. Settlement will not occur until an investor’s funds have cleared and we accept the investor as a shareholder.
By executing the subscription agreement and paying the total purchase price for the Offered Shares subscribed, each investor agrees to accept the terms of the subscription agreement and attests that the investor meets certain minimum financial standards. (See “State Qualification and Investor Suitability Standards” below).
An approved trustee must process and forward to us subscriptions made through IRAs, Keogh plans and 401(k) plans. In the case of investments through IRAs, Keogh plans and 401(k) plans, we will send the confirmation and notice of our acceptance to the trustee.
Minimum Purchase Requirements
You must initially purchase at least $5,000 of the Offered Shares in this offering. If you have satisfied the minimum purchase requirement, any additional purchase must be in an amount of at least $1,000.
State Law Exemption and Offerings to “Qualified Purchasers”
The Offered Shares are being offered and sold only to “qualified purchasers” (as defined in Regulation A under the Securities Act). As a Tier 2 offering pursuant to Regulation A under the Securities Act, this offering will be exempt from state “Blue Sky” law review, subject to certain state filing requirements and anti-fraud provisions, to the extent that the Offered Shares offered hereby are offered and sold only to “qualified purchasers”. “Qualified purchasers” include: (a) “accredited investors” under Rule 501(a) of Regulation D and (b) all other investors, so long as their investment in Offered Shares does not represent more than 10% of the greater of their annual income or net worth (for natural persons), or 10% of the greater of annual revenue or net assets at fiscal year-end (for non-natural persons). Accordingly, we reserve the right to reject any investor’s subscription in whole or in part for any reason, including if we determine, in our sole and absolute discretion, that such investor is not a “qualified purchaser” for purposes of Regulation A. We intend to offer and sell the Offered Shares to qualified purchasers in every state of the United States.
Issuance of Offered Shares
Upon settlement, that is, at such time as an investor’s funds have cleared and we have accepted an investor’s subscription agreement, we will issue a certificate or certificates representing such investor’s purchased Offered Shares, or otherwise instruct our transfer agent to effect book entry issuances of the Offered Shares.
Transferability of the Offered Shares
The Offered Shares will be generally freely transferable, subject to any restrictions imposed by applicable securities laws or regulations.
Advertising, Sales and Other Promotional Materials
In addition to this Offering Circular, subject to limitations imposed by applicable securities laws, we expect to use additional advertising, sales and other promotional materials in connection with this offering. These materials may include information relating to this offering, articles and publications concerning industries relevant to our business operations or public advertisements and audio-visual materials, in each case only as authorized by us. In addition, the sales material may contain certain quotes from various publications without obtaining the consent of the author or the publication for use of the quoted material in the sales material. Although these materials will not contain information in conflict with the information provided by this Offering Circular and will be prepared with a view to presenting a balanced discussion of risk and reward with respect to the Offered Shares, these materials will not give a complete understanding of our company, this offering or the Offered Shares and are not to be considered part of this Offering Circular. This offering is made only by means of this Offering Circular and prospective investors must read and rely on the information provided in this Offering Circular in connection with their decision to invest in the Offered Shares.
| 19 |
General
Our authorized capital stock consists of (a) 325,000,000 shares of common stock, $.0001 par value per share; and (b) 28,000 shares of preferred stock, $.0001 par value per share, of which 28,000 shares have been designated, as follows: (1) Series A-1 Supermajority Voting Preferred Stock, 1,000 shares authorized; (2) Series B-1 Convertible Redeemable Preferred Stock, 25,000 shares authorized; and (3) Series C-1 Convertible Redeemable Preferred Stock, 2,000 shares authorized.
Our Board of Directors has determined to increase the number of authorized shares of our common stock. However, to date, there has been no determination as to the amount of such share increase.
As of the date of this Offering Circular, there were (w) 234,953,286 shares of our common stock issued and outstanding, held by 78 holders of record; (x) 1,000 shares of Series A-1 Supermajority Voting Preferred Stock issued and outstanding, held by one (1) holder of record; (y) 14,950 shares of Series B-1 Convertible Redeemable Preferred Stock issued and outstanding, held by two (1) holders of record; and (z) 225 shares of Series C-1 Convertible Redeemable Preferred Stock issued and outstanding, held by two holders of record.
Common Stock
General. The holders of our common stock currently have (a) equal ratable rights to dividends from funds legally available therefore, when, as and if declared by our Board of Directors; (b) are entitled to share ratably in all of our assets available for distribution to holders of common stock upon liquidation, dissolution or winding up of the affairs of our company; (c) do not have preemptive, subscriptive or conversion rights and there are no redemption or sinking fund provisions or rights applicable thereto; and (d) are entitled to one non-cumulative vote per share on all matters on which shareholders may vote. Our Bylaws provide that, at all meetings of the shareholders for the election of directors, a plurality of the votes cast shall be sufficient to elect. On all other matters, except as otherwise required by Nevada law or our Articles of Incorporation, as amended, a majority of the votes cast at a meeting of the shareholders shall be necessary to authorize any corporate action to be taken by vote of the shareholders.
Non-cumulative Voting. Holders of shares of our common stock do not have cumulative voting rights, which means that the holders of more than 50% of the outstanding shares, voting for the election of directors, can elect all of the directors to be elected, if they so choose, and, in such event, the holders of the remaining shares will not be able to elect any of our directors.
In addition, all of the issued and outstanding shares of Series A-1 Supermajority Voting Preferred Stock are owned by Epizon Limited (see Note 5 to the ownership table under “Security Ownership of Certain Beneficial Owners and Management” for information concerning beneficial ownership and control of Epizon Limited). Epizon Limited, thus, controls all corporate matters of our company. (See “Security Ownership of Certain Beneficial Owners and Management” and “Certain Relationships and Related Transactions”).
Pre-emptive Rights. As of the date of this Offering Circular, no holder of any shares of our capital stock has pre-emptive or preferential rights to acquire or subscribe for any unissued shares of any class of our capital stock not otherwise disclosed herein.
Preferred Stock
Series A-1 Supermajority Voting Preferred Stock. We have authorized 1,000 shares of $0.0001 par value (stated value $10) Series A-1 Supermajority Voting Preferred Stock of which 1,000 are issued and outstanding as of the date of this Offering Circular. So long as one share of Series A-1 Supermajority Voting Preferred Stock is outstanding, the outstanding shares of the Series A-1 Supermajority Voting Preferred Stock shall have the number of votes, in the aggregate, equal to 51% of all votes entitled to be voted at any shareholder meeting. The shares of Series A-1 Supermajority Voting Preferred Stock have no rights to receive dividends and liquidation rights are equal to the stated value per share.
Series B-1 Convertible Redeemable Preferred Stock. We have authorized 25,000 shares of $0.0001 par value (stated value $1,000) Series B-1 Convertible Redeemable Preferred Stock of which 14,950 shares are issued and outstanding as of the date of this Offering Circular. These shares have no voting rights, dividends on these shares shall accrue at the rate of 5% of the stated value per share and liquidation rights are equal to the stated value per share. These shares are convertible into InnovaQor’s Common Stock based on the stated value at a conversion price equal to 90% of the average closing price of the Common Stock on the 10 Trading Days immediately prior to the Conversion Date but in any event no less than the par value of the Common Stock. The Series B-1 Convertible Redeemable Preferred Stock may not be converted prior to the first anniversary of its issuance except with the consent of the holders of a majority of the then outstanding shares, if any, of the Series A-1 Supermajority Voting Preferred Stock. No conversion can be made to the extent the holder’s beneficial interest (as defined pursuant to the terms of the Series B-1 Convertible Redeemable Preferred Stock) in the common stock of InnovaQor would exceed 4.99%. These shares are redeemable at the option of InnovaQor at their stated value plus declared and unpaid dividends.
| 20 |
Series C-1 Convertible Redeemable Preferred Stock. We have authorized 2,000 shares of $0.0001 par value (stated value $1,000) Series C-1 Convertible Redeemable Preferred Stock of which 225 share are issued and outstanding as of the date of this Offering Circular. These shares have no voting rights, dividends on these shares shall accrue at the rate of 10% of the stated value per share and liquidation rights are equal to the stated value per share. These shares are convertible into InnovaQor’s Common Stock based on the stated value at a conversion price equal to 90% of the average closing price of the Common Stock on the 10 Trading Days immediately prior to the Conversion Date but in any event no less than the par value of the Common Stock. The Series C-1 Convertible Redeemable Preferred Stock may not be converted prior to the first anniversary of its issuance except with the consent of the holders of a majority of the then outstanding shares, if any, of the Series A-1 Supermajority Voting Preferred Stock. No conversion can be made to the extent the holder’s beneficial interest (as defined pursuant to the terms of the Series C-1 Convertible Redeemable Preferred Stock) in the common stock of InnovaQor would exceed 4.99%. These shares are redeemable at the option of InnovaQor at their stated value plus declared and unpaid dividends.
Anti-takeover Provisions in Our Articles of Incorporation and Bylaws
Authorized But Unissued Preferred Stock. As of the date of this Offering Circular, there is no existing obligation that involves the issuance of preferred stock or common stock (except upon conversion of outstanding shares of preferred stock).
Amending Our Bylaws. Our Articles of Incorporation authorize our Board of Directors to adopt, amend or repeal our bylaws.
Special Meetings of Shareholders. Our Articles of Incorporation provide that special meetings of our shareholders may be called at any time only by (1) the Board of Directors, (2) any two directors, (3) the Chairperson of the Board or (4) the Chief Executive Officer or the President together with one non-employee director. Shareholders may not call a special meeting for any purpose.
Filling Board Vacancies. Our Articles of Incorporation provide that, subject to the rights, if any, of the holders of shares of preferred stock then outstanding, newly created directorships resulting from any increase in the number of directors or any vacancy on the Board of Directors resulting from death, resignation, disqualification, removal or other cause shall be filled solely by the affirmative vote of a majority of the remaining directors then in office, even though less than a quorum, or by a sole remaining director.
Removal of Directors. The provisions of our bylaws may make it difficult for our shareholders to remove one or more of our directors. Our bylaws provide that the entire Board of Directors, or any individual director, may be removed from office at any special meeting of shareholders called for such purpose by vote of the holders of two-thirds of the voting power entitling the shareholders to elect directors in place of those to be removed. Our bylaws also provide that when the holders of the shares of any class or series voting as a class or series are entitled to elect one or more directors, any director so elected may be removed only by the applicable vote of the holders of the shares of that class or series.
Board Action Without Meeting. Our bylaws provide that the Board may take action without a meeting if all the members of the Board consent to the action in writing. Board action through consent allows the Board to make swift decisions, including in the event that a hostile takeover threatens current management.
Voting. Our shares of Series A-1 Supermajority Voting Preferred Stock have majority voting rights, which means they represent 51% of the voting rights of all classes of shares combined.
Authorized but Unissued Shares. Our authorized but unissued shares of common stock and preferred stock will be available for future issuance. We may use additional shares for a variety of purposes, including future public offerings to raise additional capital, to fund acquisitions and as employee compensation. The existence of authorized but unissued shares of common stock and preferred stock could render more difficult or discourage an attempt to obtain control of InnovaQor by means of a proxy contest, tender offer, merger or otherwise.
Listing. Our common stock is quoted on the OTC Pink under the symbol VMCS. We have applied to FINRA for a new trading symbol.
Dividend Policy
We have never declared or paid any dividends on our common stock. We currently intend to retain future earnings, if any, to finance the expansion of our business. As a result, we do not anticipate paying any cash dividends in the foreseeable future.
| 21 |
Transfer Agent
We have retained the services of Olde Monmouth Stock Transfer Co., Inc., 200 Memorial Parkway, Atlantic Heights, New Jersey 07716, as the transfer agent for our common stock. Olde Monmouth Stock Transfer’s website is located at: www.oldemonmouth.com. No information found on Olde Monmouth Stock Transfer’s website is part of this Offering Circular.
From time to time, InnovaQor may be involved in a variety of claims, lawsuits, investigations and proceedings related to contractual disputes, employment matters, regulatory and compliance matters, intellectual property rights and other litigation arising in the ordinary course of business. InnovaQor operates in a highly regulated industry which may inherently lend itself to legal matters. Management is aware that litigation has associated costs and that results of adverse litigation verdicts could have a material effect on InnovaQor’s consolidated financial position or results of operations. Management, in consultation with legal counsel, has addressed known assertions and predicted unasserted claims below.
P2P Staffing Corp. received a judgment against HTS during 2018 in the amount of $58,784 plus accrued interest and court costs for amounts owed. As of June 30, 2022 and December 31, 2021, $10,464 was outstanding and owed for this judgment and included in accounts payable at each respective balance sheet date.
Two former employees of CollabRx, Inc., one of the acquired subsidiaries, filed suits in a California state court against the former Parent, Rennova and CollabRx, Inc., in connection with amounts claimed to be owed under their respective employment agreements with CollabRx, Inc. One former employee received a judgment for approximately $253,000, which Rennova has paid in full. The other former employee received a judgment for approximately $173,000.
ClinLab, Medical Mime and HTS, as well as the former Parent, Rennova and many of its subsidiaries, were defendants in a case filed in Broward County Circuit Court by TCA Global Credit Master Fund, L.P. The plaintiff alleged a breach by Medytox Solutions, Inc. of its obligations under a debenture and claimed damages of approximately $2,030,000 plus interest, costs and fees. In May 2020, the SEC appointed a Receiver to close down the TCA Global Credit Master Fund, L.P. The other entities were sued as alleged guarantors of the debenture. In September 2021, the parties entered into a settlement agreement with the Receiver to pay $500,000 as full and final settlement of all claims in this matter, which amount has now been paid as of April 2022.
CTI Consulting LLC filed suit against HTS in September 2021, claiming approximately $45,000 as owed for services provided. The Company has agreed to pay $5,000 per month until the obligation is satisfied.
On June 14, 2018, Techlogix, Inc. obtained a judgment in the Massachusetts Superior Court for Middlesex County in the amount of $71,223 against HTS and Rennova. This amount was paid by Rennova and a Satisfaction of Judgement was filed by Techlogix, Inc. on December 8, 2020.
Overview
Our company, InnovaQor, Inc., a Nevada corporation, provides information technology solutions and services to healthcare and laboratory customers in the United States. Our goal is to develop and deliver a technology-based social media and communication platform to a broad range of healthcare professionals and businesses using a subscription revenue model with added value bolt on services. The Company, through an acquisition that closed on June 25, 2021, has a number of fully developed products and services which it offers through six wholly-owned subsidiaries that provide medical support services primarily to clinical laboratories, corporate operations, rural hospitals, physician practices and behavioral health/substance abuse centers.
The Company has the following wholly-owned subsidiaries, which it purchased on June 25, 2021: Health Technology Solutions, Inc., Medical Mime, Inc., ClinLab, Inc., Advanced Molecular Services Group, Inc. (“AMSG”), Genomas, Inc. and CollabRx, Inc. These subsidiaries provided products and services to 36 and 61 customers in the United States and generated $468,883 and $528,624 (including $237,551 and $185,892 from a related party) in net revenues during the years ended December 31, 2021 and 2020, respectively. Net revenues amounted to $94,511 and $104,676 (including $47,788 and $62,316 from a related party) for the three months ended June 30, 2022 and 2021, from 18 and 25 customers in the United States, respectively. Net revenues amounted to $191,327 and $216,941 (including $102,267 and $124,632 from a related party) for the six months ended June 30, 2022 and 2021, from 18 and 25 customers in the United States, respectively.
| 22 |
Health Technology Solutions, Inc. (“HTS”): HTS provides virtual chief information officer (vCIO), IT managed services and data analytics dashboards to our subsidiaries and outside medical service providers. HTS operates from the corporate offices in West Palm Beach, Florida.
Medical Mime, Inc. (“Mime”): Mime was formed on May 9, 2014. It specializes in electronic health records (EHR) software and subscription services for the behavioral health and rehabilitation market segments. It currently serves nine behavioral health/substance abuse facilities.
ClinLab, Inc. (“ClinLab”): ClinLab develops and markets laboratory information management systems to mid-size clinical laboratories. It currently services 13 clinical laboratories across the country.
AMSG owns CollabRx, Inc. (“CollabRx”) and Genomas, Inc. (“Genomas”), each of which is an inactive operation.
Genomas operated a diagnostics lab until December 31, 2019, and was focused solely on the pharmacogenomics technology and platform, MedTuning, to interpret diagnostics outcomes and translate these outcomes into easily usable information to indicate the effectiveness of medications for a patient. This solution would require minimum effort to be back in operation. CollabRx owns a technology platform and database for interpreting diagnostics outcomes from cancer patients that could match the result to known treatments and or clinical trials. This solution has been dormant for a number of years and to be viable in the marketplace will require updates to the technology and the database.
Each of the subsidiaries is wholly owned by the Company and complements each other, allowing for cross selling of products and services. The Company believes the current solutions will become an added value option to a technology-based social media communication platform to a broad range of healthcare professionals and businesses using a subscription revenue model with added value bolt on services the Company plans to develop.
In the coming year we plan to develop, acquire or license and offer a telehealth solution through corporate partnerships in the emerging health technology sector.
Company History
The Company was originally incorporated in the State of Nevada on September 7, 1999, under the name Ancona Mining Corporation.
The Company’s name was changed to VisualMED Clinical Solutions Corporation on November 30, 2004, from Ancona Mining Corporation.
The Company’s name was changed to InnovaQor, Inc. on September 8, 2021, from VisualMED Clinical Solutions Corporation.
VisualMED was a medical information company that used technology to assist physicians and nurses streamline the mass of patient information in a coherent and usable manner. Its clinical information systems were designed for use in hospitals, healthcare delivery organizations and regional and national healthcare authorities. In response to changes in the marketplace, the Company then sought to take its applications originally created for clinicians and make them available to patients and individuals concerned about their health. As part of this process the Company partnered with various consultants to consider the medical applications, develop a marketing strategy and investigate how best to transition its existing applications to upgraded versions, including integrating artificial intelligence for data assessment and outcomes. With the onset of the COVID-19 pandemic, however, it became apparent that this business opportunity would require more capital, management capability and time than what was available to the Company.
In late 2020, the majority of shareholders and the Board of Directors charged management of VisualMED to find a new business opportunity for the Company that would allow it to leverage its healthcare, software and IT experience. At the beginning of 2021, the Company initiated measures that would facilitate a new opportunity for the Company. Subsequently, in May 2021, then CEO Gerard Dab entered into an agreement with and engaged the services of Epizon Limited (“Epizon”), a Nassau, Bahamas, based management consulting company specializing in the provision of management services to secure financing and opportunities for growth. Seamus Lagan, the Chief Executive Officer of Rennova Health, Inc. (“Rennova”), the company we ultimately completed a transaction with, is also the managing director of Epizon.
The objective of the agreement with Epizon was to help VisualMED find a new opportunity in its core healthcare technology business. The Company needed to find and develop new products that would be more relevant for a changing healthcare marketplace. Epizon was engaged to assist VisualMED with its capital structure, and to look for new business opportunities and/or acquisitions that could result in improved shareholder value. The terms of the agreement with Epizon called for the transfer to Epizon of 1,000 shares of Series A-1 Supermajority Voting Preferred Stock (the “Series A-1 Preferred Stock”), with a stated value of $10.00 each, personally owned by Gerard Dab, on the successful completion of a transaction as defined in the agreement. It was determined that an agreement with Rennova was the most viable opportunity available to VisualMED. The conditions of the Epizon agreement were met and the transfer of shares of Series A-1 Preferred Stock was completed. This transfer resulted in a change of voting control of VisualMED, as the Series A-1 Preferred Stock, in the aggregate, has the right to the number of votes equal to 51% of the votes entitled to be cast at a meeting or to vote by written consent. As the owner of the Series A-1 Preferred Stock, Epizon will be able to exercise control over all matters submitted for stockholder approval.
| 23 |
In May 2021, VisualMED entered into an acquisition agreement with Rennova to acquire certain subsidiaries owned by Rennova. This has been accounted for as a reverse acquisition in the accompanying financial statements.
On June 25, 2021, VisualMED closed the acquisition agreement with Rennova. These subsidiaries are Health Technology Solutions, Inc., Medical Mime, Inc., ClinLab, Inc., Advanced Molecular Services Group, Inc., Genomas, Inc. and CollabRx, Inc., and combined are referred to herein as HTS and AMSG (the “HTS Group”).
Products offered by the acquired entities include vCIO services, IT managed services, healthcare finance and operational business intelligence analytics dashboards, an EHR (electronic health records software), an LIS (laboratory information system), and a lab ordering and reporting software. The CollabRx and Genomas subsidiaries provided actionable data analytics and reporting for oncologists to enhance cancer diagnoses and treatment and PhyzioType Systems for DNA-guided management and prescription of drugs, respectively. These subsidiaries are not currently operating.
The Company operates its subsidiaries under the following structure:

In consideration for the shares of HTS and AMSG and the elimination of inter-company debt between Rennova and HTS and AMSG, the Company issued 14,000 shares of its Series B-1 Convertible Redeemable Preferred Stock to Rennova. The number of shares of Series B-1 Preferred Stock was subject to a post-closing adjustment which resulted in 950 additional shares of Series B-1 Preferred Stock due Rennova which were issued in September 2021. Each share of Series B-1 Preferred Stock has a stated value of $1,000 and is convertible into that number of shares of the Company’s common stock equal to the product of the stated value divided by 90% of the average closing price of the common stock during the 10 trading days immediately prior to the conversion date. Conversion of the Series B-1 Preferred Stock, however, is subject to the limitation that no conversion can be made to the extent the holder’s beneficial interest (as defined pursuant to the terms of the Series B-1 Preferred Stock) in the common stock of the Company would exceed 4.99%. The shares of Series B-1 Preferred Stock may be redeemed by the Company upon payment of the stated value of the shares plus any accrued declared and unpaid dividends. In addition, prior to the acquisition the Company’s former CEO, Gerard Dab, forgave $300,000 owed to him by the Company in exchange for the issuance of 1,000 shares of Series A-1 Preferred Stock. These shares of Series A-1 Preferred Stock were subsequently transferred to Epizon. Mr. Dab also forgave another $200,000 owed to him from the Company in exchange for 200 shares of Series C-1 Convertible Redeemable Preferred Stock (the “Series C-1 Preferred Stock”) with each share having a stated value of $1,000 and convertible into that number of shares of common stock equal to the product of the stated value divided by 90% of the average closing price of the common stock during the 10 trading days immediately prior to the conversion date. Conversion of the Series C-1 Preferred Stock is also subject to a similar 4.99% beneficial ownership limitation. Shares of the Series B-1 Preferred Stock and Series C-1 Preferred Stock were not convertible prior to the first anniversary of their issuance without the consent of the holders of a majority of the then outstanding shares, if any, of the Series A-1 Preferred Stock. Because these shares of Series B-1 Preferred Stock and Series C-1 Preferred Stock are convertible, at the option of the holder, into a variable number of common shares based solely on a fixed dollar amount (stated value) known at issuance of the shares, they have been recorded as a long-time liability at the date of issuance in accordance with ASC 480, Distinguishing Liabilities from Equity.
| 24 |
The following table represents the Company’s issued shares at June 30, 2022:
| Common Stock | 234,953,286 | |||
| Series A-1 Supermajority Voting Preferred Stock | 1,000 | |||
| Series B-1 Convertible Redeemable Preferred Stock | 14,950 | |||
| Series C-1 Convertible Redeemable Preferred Stock | 225 |
Subsidiaries
The Company has six wholly-owned subsidiaries that provide medical support services primarily to clinical laboratories, corporate operations, rural hospitals, physician practices and behavioral health/substance abuse centers.
Health Technology Solutions, Inc. (“HTS”): HTS provides vCIO, IT managed services and data analytics dashboards to our subsidiaries and outside medical service providers. HTS operates from the corporate offices in West Palm Beach, Florida.
Medical Mime, Inc. (“Mime”): Mime was formed on May 9, 2014. It specializes in electronic health records (her) software and subscription services for the behavioral health and rehabilitation market segments. It currently serves nine behavioral health/substance abuse facilities.
ClinLab, Inc. (“ClinLab”): ClinLab develops and markets laboratory information management systems to mid-size clinical laboratories. It currently services 12 clinical laboratories across the country.
AMSG owns CollabRx, Inc. (“CollabRx”) and Genomas, Inc. (“Genomas”), each of which is an inactive operation. Genomas operated a diagnostics lab until December 31, 2019, and was focused solely on the pharmacogenomics technology and platform, MedTuning, to interpret diagnostics outcomes and translate these outcomes into easily usable information to indicate the effectiveness of medications for a patient. This solution would require minimum effort to be back in operation CollabRx owns a technology platform and database for interpreting diagnostics outcomes from cancer patients that could match the result to known treatments and or clinical trials. This solution has been dormant for a number of years and to be viable in the marketplace will require updates to the technology and the database.
Each of the subsidiaries is wholly owned by the Company and complements each other, allowing for cross selling of products and services. The Company believes the current solutions will become an added value option to a technology-based social media communication platform to a broad range of healthcare professionals and businesses using a subscription revenue model with added value bolt on services the Company plans to develop.
In the coming year we plan to develop, acquire or license and offer a telehealth solution through partnerships in the emerging health technology sector.
Company Information
The address of our principal executive offices is 400 S. Australian Avenue, Suite 800, West Palm Beach, Florida 33401 and our telephone number at that location is (561) 421-1900.
Our website is www.innovaqor.com. The information contained on, or that may be obtained from, our website is not a part of this offering circular. We have included our website address in this information statement solely as an inactive textual reference.
Terms of the Recent Acquisition
Background. On June 25, 2021, the Company completed the acquisition agreement with Rennova, and acquired 100% ownership of certain subsidiaries of Rennova. The acquired businesses are now the main business of the Company.
Reasons for the Acquisition. The previous business model of the Company had not generated revenue for over five years. The Board of Directors and majority shareholders had determined the Company should pursue other opportunities for acquisition of technology and services that were similar in nature to the existing business of the Company. The Company had limited resources of cash and management and believed that an acquisition that could be completed without cash and that had its own management team would provide the best opportunity for a successful closing. The Company believes that the acquired assets and new management team create a new opportunity for the Company in a sector in which the Company’s solutions and services are in demand and should generate profitable revenue. The Company believes the acquisition brings the following benefits for shareholders:
| ● | Enhanced strategic and management focus – The acquisition will provide the Company with a well-established and accomplished management team to more effectively pursue its distinct operating priorities and strategies and enable the management to quickly and efficiently make decisions and concentrate efforts on the unique needs of each business and pursue opportunities for long-term growth and profitability. In this way, the Company’s management will be able to focus exclusively on its IT products and services business and productize its services to third parties. |
| 25 |
| ● | Direct access to capital markets – The acquisition provides the Company with a variety of existing product lines, some already generating revenue. These constitute a firm basis for supporting the Company’s business expansion. This should also mean that the Company will achieve better access to the capital markets to support a credible expansion plan. |
| ● | Alignment of incentives with performance objectives – The acquisition will facilitate incentive compensation arrangements for employees more directly tied to the performance of the business, and may enhance employee hiring and retention by, among other things, improving the alignment of management and employee incentives with performance and growth objectives. |
The Company cannot assure you that, as a result of the acquisition, any of the benefits described above or otherwise will be realized to the extent anticipated or at all and would highlight that the acquisition adds increased risk to the Company with the following;
| ● | Increased costs – the Company will assume increased costs related to the business operations and development plan and will see an immediate increase in legal and accounting costs associated with the acquisition and the Company’s plans to become fully reporting and compliant with the SEC reporting requirements. If the Company fails to raise additional capital it may fail to deliver its business plan. | |
| ● | The Company may experience disruptions to the business of the acquired entities as a result of the acquisition. The acquired entities had enjoyed revenue and financial assistance from related parties under its previous structure. There is no guarantee that these revenues can be retained and the acquired entities will no longer be able to rely on the support and services received prior to acquisition. | |
| ● | One-time costs of the acquisition may be significant. The Company will incur costs in connection with the acquisition that may include accounting, tax, legal and other professional services costs, recruiting, and relocation costs associated with hiring or reassigning personnel, costs related to establishing a new brand identity in the marketplace and costs to separate information systems. | |
| ● | Inability to realize anticipated benefits of the acquisition – the Company may not achieve the anticipated benefits of the acquisition for a variety of reasons, including, among others: following the acquisition, the Company may be more susceptible to market fluctuations and other adverse events. | |
The prior Board of Directors concluded that the potential benefits of the acquisition outweighed the risks and concluded that it was in the best interest of the Company and its shareholders to complete the acquisition as described.
Business
InnovaQor has expertise in the areas of IT involving the design, development, creation, use and maintenance of information systems for the healthcare industry. These applications and systems will continue to improve patient care, lower costs, increase efficiency, reduce errors and improve patient outcomes. In addition, these applications and systems will accelerate and maximize reimbursements for healthcare providers.
InnovaQor also recognizes the future in interoperability (sharing data between multiple various health IT systems), telemedicine (the ability to access and interact with health data and practitioners/patients via mobile devices) and the increasing use of blockchain technologies to protect access to medical records.
We intend to develop, acquire or license and offer a telehealth solution through corporate partnerships in the emerging health technology sector.
Existing products offered by the Company’s subsidiaries are as follows:
“M2Select” is a custom built, cloud based, electronic health record which meets the needs of substance abuse treatment and behavioral health providers. M2Select’s specialized clinical workflow provides intuitive prompts for symptoms and enables you to quickly select problems and create master treatment plans with goals, objectives, and interventions. M2Select provides best-in-class patient lifecycle management for Behavioral Health/Substance Abuse (BH/SA) treatment centers. From pre-admission to billing and aftercare, M2Select is an electronic health record and patient management software that seamlessly integrates into the natural workflow of day-to-day operations.
| 26 |
“M2Pro” is a custom built, cloud based, electronic health record for ambulatory physician practices that meets meaningful use stage 2 and no further. Its unique dictation services further automate the workflow process for physicians allowing them to focus on their continuum of patient care.
“ClinLab” is a turnkey client/server lab information system for mid-range laboratories. ClinLab supports interfaces to all major reference labs and the ClinLab team can provide an interface to any system with that capability. ClinLab also features an optional EHR package which enables interfacing with the most popular EHR systems allowing lab test results to integrate seamlessly into a provider’s EHR for an improved patient record and to fulfill the federal government requirements.
“Qira” is our healthcare business analytics service powered by PowerBI. It is a culmination of healthcare financial and revenue cycle management plus clinical operations oversight needs. It aggregates data from multiple healthcare systems to produce a single source business intelligence tool with executive level daily briefing to deep dive operational management of claims and operational efficiencies. There are many other analytical services available that customize solutions but none that has a proven template for success. Our competitive advantage comes from having created these tools to identify the deficiencies in the real world for the former parent Rennova from its former national laboratory operations to its more recent rural hospitals.
“vCIO Services”. Based on the skills and experience inherent within InnovaQor and resulting from work undertaken on behalf of the former parent, Rennova, InnovaQor offers a range of CIO services centered on our ability to link IT systems to business objectives combined with our knowledge of technology trends likely to impact our sector. The CIO services would include (but not be limited to):
| ● | Program and Project Management | |
| ● | Business Continuity and Disaster Recovery | |
| ● | Security Services | |
| ● | Business Intelligence and Analytics | |
| ● | Network Infrastructure Management | |
| ● | Helpdesk Provision |
“MedTuning” is the technology and platform owned by Genomas. It utilized proprietary biomarkers, treatment algorithms, and a web-based interactive physician portal delivery system to provide clinical decision support for physicians and personalized drug treatment for patients. Products were DNA-guided to improve the therapeutic benefit of widely used prescription drugs while also reducing the risk of significant side effects for patients.
Medical Informatics: Our technology platform, proprietary algorithms and physician interface portal can be extended to a wide range of drug categories.
Research and Development: Technology platform applicable to numerous disease states; current pipeline in mental health, pain management, cardiovascular and diabetes.
“Advantage” is a proprietary HIPAA compliant software developed to eliminate the need for paper requisitions by providing an easy to use and efficient web-based system that lets customers securely place lab orders, track samples and view test reports in real time from any web-enabled laptop, notepad or smart phone.
Brands
We intend to trademark both InnovaQor and its products and services, i.e. ClinLab, M2Select, Qira, vCIO and Health Technology Solutions.
Sales
The HTS Group provided products and services to 36 and 61 customers in the United States and generated $468,883 and $528,624 in net revenues during the years ended December 31, 2021 and 2020, respectively. Included in net revenues were sales made to the former parent and related entities of $237,551 and $185,892 for the years ended December 31, 2021 and 2020, respectively. Net revenues amounted to $94,511 and $104,676 for the three months ended June 30, 2022 and 2021 to 18 and 25 customers in the United States, respectively. Included in net revenues were sales to the former parent and related entities of $47,788 and $62,316 for the three months ended June 30, 2022 and 2021, respectively. Net revenues amounted to $191,327 and $216,941 for the six months ended June 30, 2022 and 2021 to 18 and 25 customers in the United States, respectively. Included in net revenues were sales to the former parent and related entities of $102,267 and $124,632 for the six months ended June 30, 2022 and 2021, respectively.
| 27 |
Distribution
InnovaQor intends to sell its Health Technology Solutions, Medical Mime and ClinLab products and services directly to customers through internal sales and digital marketing. InnovaQor intends to identify strategic partnerships that sell into the sectors it is targeting. InnovaQor intends to promote these products and services to the strategic partnerships’ existing clientele coming to agreement on a recurring revenue based on cash collected for closed sales of these products and services.
Competitive Position
The healthcare software, IT and vCIO consulting services industry is extremely competitive, highly fragmented, and subject to rapid change. The industry includes a large number of participants with a variety of skills and industry expertise, including other strategy, business operations, technology, technical advisory firms, regional and specialty consulting firms, and the internal professional resources of organizations. We compete with a large number of service and technology providers in all of our segments. Our competitors often vary, depending on the particular practice area. We expect to continue to face competition from new entrants.
We believe the principal competitive factors in our market include reputation, the ability to attract and retain top talent, and the capacity to manage engagements effectively to drive high value to clients. There is also competition on price, although to a lesser extent due to the criticality of the issues that many of our services address. Our competitors often have a greater geographic footprint, a broader international presence, and more resources than we do, but we believe that our industry experience and reputation, ability to deliver meaningful client results, and balanced portfolio of services enable us to compete favorably in the consulting marketplace.
Our rehab EHR product, Medical Mime, is a main competitor in its sector and our immediate competition is provided by KIPU, BestNotes, Zencharts, Sunwave, and TherapyNotes. Our competitive advantage is a system developed with and for facilities practicing in this sector along with customized reports and forms. Our system offers partially automated implementation and fully automated billing files that restrict billing until all required documentation is available while flagging operational deficiencies.
Our LIS, ClinLab, is a small player in its sector and our immediate competitors are LabDaq, Schuyler House and RelayMed. Our competitive advantage is a select feature set and affordability.
Our vCIO services are just launching and have the experience of being the internal IT team for the former parent company, Rennova. With a 10-year experience in providing complete services, consulting, project management, software management, vendor management and network engineering, vCIO will specialize in healthcare facilities.
Qira is our healthcare business analytics tool powered by PowerBI. It is a culmination of healthcare financial and revenue cycle management plus clinical operations operational oversight needs. It aggregates data from multiple healthcare systems to produce a single source business intelligence tool with executive level daily briefing to deep dive operational management of claims and operational efficiencies. There are many other analytical services available that customize solutions but none that has a proven template for success. Our competitive advantage comes from having created these tools to identify the deficiencies in the real world for the former parent Rennova from its former national laboratory operations to its more recent rural hospitals. This product easily pays for itself as it immediately eliminates the need for accountants’ monthly delivery of numbers that can cost upwards of $25,000 a month.
Research and Development
The industries and market segments in which we plan to operate and compete are subject to rapid technological developments, evolving industry standards, changes in customer requirements and competitive new products and features. As a result, we believe our success, in part, will depend on our ability to build and enhance our products in a timely and efficient manner and to develop and introduce new products that meet our clients’ needs and help our clients reduce their total cost of operation. To achieve these objectives, we plan to make research and development investments through internal and third-party development activities, third-party licensing agreements and potentially through joint ventures and acquisitions.
Research and Intellectual Property. Our future success and ability to compete will depend on our ability to develop and maintain our intellectual property and proprietary technology and to operate without infringing on the proprietary rights of others. Software products are generally licensed to customers on a non-exclusive basis for internal use in a customer’s organization. We plan to also grant rights in intellectual property that we plan on developing or acquiring to third parties to allow them to market certain of our future products on a non-exclusive or limited-scope exclusive basis for an application of such product or to a specific geographic region.
InnovaQor plans to protect its intellectual property in its subsidiaries through a combination of trademarks and copyrights in the coming year. InnovaQor will evaluate the possibility of acquiring or developing patents that are related to healthcare services and products.
Our IP strategy encompasses protection on composition of matter and method for DNA markers, marker ensembles, and predictive biostatistical algorithms
| 28 |
Platform Technology
Trademarks and Copyrights. U.S. Copyright (Registration Number VA 1-797-692): Personalized Health Portal with design, user interface and algorithm.
While we believe our intellectual property will be an asset, and our ability to maintain and protect our intellectual property rights is important to our success, we do not anticipate that our business will be materially dependent on any patent, trademark, license, or other intellectual property right.
Employees
As of the date of this offering circular, we have six employees, four of whom are working on maintenance and customer service of our existing products. We expect to grow with a focus on sales and business development eventually expanding our technical team to support the growth. We plan to hire a team of employees and contractors to deliver on the goal of developing and delivering a technology-based communication platform to a broad range of healthcare professionals and businesses using a subscription revenue model with added value bolt on services.
Cyclical Nature of the Business
We have found that our business is not very cyclical but it does exhibit certain seasonality around holiday periods.
Regulatory Matters
The healthcare industry is subject to extensive government regulation, most notably the Health Insurance Portability and Accountability Act (HIPAA) and Protected Health Information (PHI).
HIPAA helps protect the privacy of patient information by:
| ● | Providing the ability to transfer and continue health insurance coverage for millions of American workers and their families when they change or lose their jobs; |
| ● | Reducing health care fraud and abuse; |
| ● | Mandating industry-wide standards for health care information on electronic billing and other processes; and |
| ● | Requiring the protection and confidential handling of protected health information |
PHI is a HIPAA Privacy Rule that provides federal protections for personal health information held by covered entities and gives patients an array of rights with respect to that information. At the same time, the Privacy Rule is balanced so that it permits the disclosure of personal health information needed for patient care and other important purposes.
Although the standards are challenging, we believe that our products are compliant with HIPAA and PHI regulations. Nonetheless, our Company could be adversely affected if a third party is impacted by HIPAA or PHI related software defects.
Properties
InnovaQor does not own any properties, and currently subleases office space from Rennova on a month to month term at a cost of approximately $6,500 a month.
Emerging Growth Company Status of InnovaQor
An emerging growth company (EGC) is any company that meets the following requirements and will lose its emerging growth status should it exceed any of these:
| ● | The company has less than $1.07 billion or more of total gross revenue in a consecutive 12-month period; |
| ● | Is within five years of its original IPO; |
| ● | The company cannot have issued more than $1 billion in non-convertible bonds within the last three years; and |
| ● | The company does not qualify as a large accelerated filer, meaning having a public float of over $700 million. |
InnovaQor is an “emerging growth company” as defined in the Jumpstart our Business Startups Act (the “JOBS Act”). As such, InnovaQor will be eligible to take advantage of certain exemptions from various reporting requirements that apply to other public companies that are not emerging growth companies, including compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and the requirements to hold a non-binding advisory vote on executive compensation and any golden parachute payments not previously approved. If InnovaQor does take advantage of some or all of these exemptions, some investors may find its common stock less attractive. The result may be a less active trading market for the common stock and its stock price may be more volatile.
| 29 |
In addition, Section 107 of the JOBS Act provides that an emerging growth company may take advantage of the extended transition period provided in Section 13(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), for complying with new or revised accounting standards, meaning that InnovaQor, as an emerging growth company, can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. It is InnovaQor’s present intention to adopt any applicable accounting standards timely. If at some time InnovaQor delays adoption of a new or revised accounting standard, our financial statements may not be comparable to those of companies that comply with such new or revised accounting standards.
MANAGEMENTS DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Cautionary Statement
The following discussion and analysis should be read in conjunction with our consolidated financial statements and related notes, beginning on page F-1 of this Offering Circular.
Our actual results may differ materially from those anticipated in the following discussion, as a result of a variety of risks and uncertainties, including those described under Cautionary Statement Regarding Forward-Looking Statements and Risk Factors. We assume no obligation to update any of the forward-looking statements included herein.
Estimates
Management’s discussion and analysis of InnovaQor’s financial condition and results of operations are based upon its consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and the related disclosure of contingent liabilities. Significant areas of estimation include estimating fair value of intangible assets acquired, the impairment of assets, accrued and contingent liabilities, and future income tax obligations (benefits), among other items. On an on-going basis, management evaluates past estimates and judgments, including those related to bad debts, accrued liabilities, derivative liabilities, and contingencies. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. InnovaQor believes the following critical accounting policies affect its more significant judgments and estimates used in the preparation of its consolidated financial statements.
Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to such differences include but are not limited to those discussed below and elsewhere in this offering circular, particularly in the section entitled “Risk Factors.”
Critical Accounting Policies
Basis of Presentation and Principles of Consolidation. The acquisition of an operating company by a non-operating public shell corporation typically results in the owners and management of the operating company having actual or effective voting and operating control of the combined company. The Securities and Exchange Commission staff considers a public shell reverse acquisition to be a capital transaction in substance, rather than a business combination. That is, the transaction is a reverse recapitalization, equivalent to the issuance of stock by the operating company for the net monetary assets of the shell corporation accompanied by a recapitalization. The accounting is similar to that resulting from a reverse acquisition, except that no goodwill or other intangible assets are recorded.
The consolidated financial statements include the accounts of only the HTS Group (the accounting acquirer) prior to June 25, 2021 and InnovaQor and the Group since the date of acquisition on June 25, 2021, with the transaction being accounted for as a recapitalization of the Group on June 25, 2021. The consolidated financial statements are prepared in conformity with U.S. GAAP and require management to make certain judgments, estimates, and assumptions. These may affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements. They also may affect the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates upon subsequent resolution of identified matters.
The accompanying condensed consolidated financial statements as of June 30, 2022 and 2021, have been derived from unaudited financial information. Intercompany accounts and transactions have been eliminated. The accompanying unaudited interim condensed consolidated financial statements have been prepared on the same basis as the annual audited financial statements and in accordance with U.S. GAAP, for interim financial information and the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim financial statements. In the opinion of management, such unaudited information includes all adjustments (consisting only of normal recurring accruals) necessary for a fair presentation of this interim information
All intercompany transactions among InnovaQor and its subsidiaries have been eliminated in the consolidation.
| 30 |
We have identified the policies discussed below as critical to our business and to the understanding of our results of operations.
Cash and Cash Equivalents. InnovaQor considers all highly liquid temporary cash investments with an original maturity of three months or less to be cash equivalents.
Fair Value Measurements. In accordance with ASC 820, “Fair Value Measurements and Disclosures,” InnovaQor applies fair value accounting for all financial assets and liabilities and non-financial assets and liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis. Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities that are required to be recorded at fair value, InnovaQor considers the principal or most advantageous market in which it would transact and the market-based risk measurements or assumptions that market participants would use in pricing the asset or liability, such as risks inherent in valuation techniques, transfer restrictions and credit risk. Fair value is estimated by applying the hierarchy, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement:
| ● | Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. | |
| ● | Level 2 applies to assets or liabilities for which there are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly, such as quoted prices for similar assets or liabilities in active markets; or quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets). | |
| ● | Level 3 applies to assets or liabilities for which fair value is derived from valuation techniques in which one or more significant inputs are unobservable, including the Company’s own assumptions. | |
The estimated fair value of financial instruments is determined by the Company using available market information and valuation methodologies considered to be appropriate.
Impairment of Long-lived Assets. InnovaQor accounts for the impairment or disposal of long-lived assets according to the Financial Accounting Standards Board’s (“FASB”) ASC 360, “Property, Plant and Equipment.” Long-lived assets are reviewed when facts and circumstances indicate that the carrying value of the asset may not be recoverable. When necessary, impaired assets are written down to estimated fair value based on the best information available. Estimated fair value is generally based on either appraised value or measured by discounting estimated future cash flows. Considerable management judgment is necessary to estimate discounted future cash flows. Accordingly, actual results could vary significantly from such estimates. As of June 30, 2022 and 2021 and December 31, 2021 and 2020, the majority of InnovaQor’s fixed assets were fully depreciated and, therefore, the carrying value of fixed assets represented fair value. Fixed assets are depreciated over lives ranging from three to seven years.
Income Taxes. The entities within the Group were included in the consolidated income tax returns of their Parent for the years ended December 31, 2020 and prior. A determination has been made by Parent’s management not to allocate any of the deferred tax assets or liabilities to the Group as of December 31, 2020 and prior. Accordingly, the Group had not provided for income taxes in the combined financial statements. The Company since June 25, 2021 uses the liability method of accounting for income taxes. Under the liability method, future tax liabilities and assets are recognized for the estimated future tax consequences attributable to differences between the amounts reported in the financial statement carrying amounts of assets and liabilities and their respective tax bases. Future tax assets and liabilities are measured using enacted or substantially enacted income tax rates expected to apply when the asset is realized or the liability settled. The effect of a change in income tax rates on future income tax liabilities and assets is recognized in income in the period that the change occurs. Future income tax assets are recognized to the extent that they are considered more likely than not to be realized. When projected future taxable income is insufficient to provide for the realization of deferred tax assets, the Company will recognize a valuation allowance.
In accordance with U.S. GAAP, the Company has determined whether a tax position of the Company is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The tax benefit to be recognized is measured as the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement. Derecognition of a tax benefit previously recognized could result in the Company recording a tax liability that would reduce net assets. The Company has determined that it has not incurred any liability for tax benefits as of June 30, 2022 and 2021 and December 31, 2021 and 2020. State income taxes will also be due on any income generated in the future
| 31 |
Revenue Recognition. We will recognize revenue in accordance with Accounting Standards Update (“ASU”) 2014-09, “Revenue from Contracts with Customers (Topic 606),” including subsequently issued updates. This series of comprehensive guidance has replaced all existing revenue recognition guidance. There is a five-step approach outlined in the standard. In determining revenue, we first identify the contract according to the scope of ASU Topic 606 with the following criteria:
| ● | Identify the contract(s) with a customer. | |
| ● | Identify the performance obligations in the contract. | |
| ● | Determine the contract price. | |
| ● | Allocate the transaction price to the performance obligations of the contract. | |
| ● | Recognize revenue when or as you satisfy a performance obligation. |
Convertible Preferred Stock. The Company classifies its Series B-1 and Series C-1 Convertible Preferred Stock as liabilities in accordance with ASC 480 Distinguishing Liabilities from Equity since the preferred stock is convertible, at the option of the holder, into a variable number of shares based solely on a fixed dollar amount (stated value) known at issuance of the preferred stock.
Basic and Diluted Net Income (Loss) Per Share. The Company computes net income (loss) per share in accordance with ASC 260, “Earnings per Share” which requires presentation of both basic and diluted earnings per share (EPS) on the face of the income statement. Basic EPS is computed by dividing net income (loss) available to common shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period. Diluted EPS gives effect to all dilutive potential common shares outstanding during the period including stock options, using the treasury stock method, and convertible preferred stock, using the if-converted method.
Diluted loss per share excludes all dilutive potential shares if their effect is antidilutive. As of June 30, 2022 and 2021 and December 31, 2021, there were approximately 2,845,200,000, 887,562,500 and 1,773,000,000 common stock equivalents, respectively, which were antidilutive due to the Company’s losses.
Recent Accounting Pronouncements. InnovaQor reviews all of the Financial Accounting Standards Board’s updates periodically to ensure InnovaQor’s compliance of its accounting policies and disclosure requirements to the Codification Topics.
Recent accounting standards issued by the FASB, including its Emerging Issues Task Force, the American Institute of Certified Public Accountants, and the SEC did not or are not believed by management to have a material impact on InnovaQor’s consolidated financial statements.
InnovaQor will continue to monitor these emerging issues to assess any potential future impact on its financial statements.
Results of Operations
Financial Presentation. The following sets forth a discussion and analysis of InnovaQor’s consolidated financial condition and results of operations for the three and six months ended June 30, 2022 and 2021, and for the fiscal years ended December 31, 2021 and 2020. This discussion and analysis should be read in conjunction with our consolidated financial statements appearing elsewhere in this filing. The following discussion contains forward-looking statements. Our actual results may differ significantly from the results discussed in such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the “Risk Factors” section.
Comparison of the Three Months ended June 30, 2022 and 2021. The following summary of our condensed consolidated results of operations should be read in conjunction with our interim consolidated financial statements for the three months ended June 30, 2022 and 2021, which are included herein.
The following table summarizes the results of our consolidated operations for the three months ended June 30, 2022 and 2021 (unaudited):
| Three Months Ended June 30, | ||||||||||||
| 2022 | 2021 | Change | ||||||||||
| Net revenues | $ | 94,511 | $ | 104,676 | $ | (10,165 | ) | |||||
| Operating expenses: | ||||||||||||
| Direct costs of revenue | 80,768 | 1,996 | (78,772 | ) | ||||||||
| General and administrative expenses | 337,695 | 272,644 | (65,051 | ) | ||||||||
| Total operating expenses | 418,463 | 274,640 | (145,819 | ) | ||||||||
| Loss from operations | (323,952 | ) | (169,964 | ) | (153,988 | ) | ||||||
| Interest expense | (17,835 | ) | (328 | ) | (17,507 | ) | ||||||
| Loss before income taxes | (341,787 | ) | (170,292 | ) | (171,495 | ) | ||||||
| Provision for income taxes | — | — | — | |||||||||
| Net loss | $ | (341,787 | ) | $ | (170,292 | ) | $ | (171,495 | ) | |||
| 32 |
Net Revenues. Net revenues were $94,511 and $104,676 for the three months ended June 30, 2022 and 2021, respectively. The reduced revenues were a result of losing customers that were acquired or went out of business and new customers not being secured because of our inability to invest in sales, marketing and delivery or in further developments of our products.
Direct Costs of Revenue. Direct costs of revenue increased by $78,772 compared to the three months ended June 30, 2021 principally due to increases in payroll and related expenses.
General and Administrative Expenses. General and administrative expenses increased by $65,051 compared to the three months ended June 30, 2021 principally due to increases in payroll and related expenses and professional fees.
Loss from Operations. Our operating loss increased by $171,495 for the three months ended June 30, 2022, when compared to a loss of $170,292 for the same period last year. The increase was due principally to the increases in our direct costs of revenue and general and administrative expenses.
Net Loss. Our net loss was $341,787 for the three months ended June 30, 2022, as compared to a net loss of $170,292 for the three months ended June 30, 2021. The $171,495 increase in net loss was principally due to the increases in our direct costs of revenue and general and administrative expenses.
Comparison of the Six Months ended June 30, 2022 and 2021. The following summary of our condensed consolidated results of operations should be read in conjunction with our interim consolidated financial statements for the six months ended June 30, 2022 and 2021, which are included herein.
The following table summarizes the results of our consolidated operations for the six months ended June 30, 2022 and 2021 (unaudited):
| Six Months Ended June 30, | ||||||||||||
| 2022 | 2021 | Change | ||||||||||
| Net revenues | $ | 191,327 | $ | 216,941 | $ | (25,614 | ) | |||||
| Operating expenses: | ||||||||||||
| Direct costs of revenue | 255,856 | 2,386 | (253,470 | ) | ||||||||
| General and administrative expenses | 520,974 | 551,203 | (30,229 | ) | ||||||||
| Total operating expenses | 776,830 | 553,589 | (23,241 | ) | ||||||||
| Loss from operations | (585,503 | ) | (336,648 | ) | (258,855 | ) | ||||||
| Interest expense | (21,007 | ) | (9,577 | ) | (11,430 | ) | ||||||
| Loss before income taxes | (606,510 | ) | (346,225 | ) | (260,285 | ) | ||||||
| Provision for income taxes | — | — | — | |||||||||
| Net loss | $ | (606,510 | ) | $ | (346,225 | ) | $ | (260,285 | ) | |||
Net Revenues. Net revenues were $191,327 and $216,941 for the six months ended June 30, 2022 and 2021, respectively. The reduced revenues were a result of losing customers that were acquired or went out of business and new customers not being secured because of our inability to invest in sales, marketing and delivery or in further development of our products.
Direct Costs of Revenue. Direct costs of revenue increased by $253,470 compared to the six months ended June 30, 2021 principally due to increases in payroll and related expenses.
General and Administrative Expenses. General and administrative expenses increased by $30,299 compared to the six months ended June 30, 2021 principally due to increases in payroll and related expenses and professional fees.
Loss from Operations. Our operating loss increased by $258,855 for the six months ended June 30, 2022, when compared to a loss of $336,648 for the same period last year. The increase was due principally to the increases in our direct costs of revenue and general and administrative expenses.
Net Loss. Our net loss was $606,510 for the six months ended June 30, 2022, as compared to a net loss of $346,225 for the six months ended June 30, 2021. The $260,285 increase in net loss was principally due to the increases in our direct costs of revenue and general and administrative expenses.
| 33 |
Comparison of the Years Ended December 31, 2021 and 2020. The following summary of our consolidated results of operations should be read in conjunction with our audited consolidated financial statements for the years ended December 31, 2021 and 2020, which are included herein.
The following table summarizes the results of our consolidated operations for the years ended December 31, 2021 and 2020:
| Year Ended December 31, | ||||||||||||
| 2021 | 2020 | Change | ||||||||||
| Net revenues | $ | 468,883 | $ | 528,624 | $ | -59,741 | ||||||
| Operating expenses: | ||||||||||||
| Direct costs of revenue | 205,649 | 5,536 | 200,113 | |||||||||
| General and administrative expenses | 1,247,687 | 1,043,242 | 204,445 | |||||||||
| Depreciation | 1,185 | 2,168 | -983 | |||||||||
| Loss from operations | (985,638 | ) | (522,322 | ) | -463,316 | |||||||
| Other (expense) income | 139,795 | (84,129 | ) | 223,924 | ||||||||
| Loss before income taxes | (845,843 | ) | (606,451 | ) | -239,392 | |||||||
| Provision for income taxes | — | — | — | |||||||||
| Net loss | $ | (845,843 | ) | $ | (606,451 | ) | $ | -239,392 | ||||
Net Revenues. Net revenues were $468,883 and $528,624 for the years ended December 31, 2021 and 2020, respectively. The reduced revenues were a result of losing customers that were acquired or went out of business and new customers not being secured because of our inability to invest in sales, marketing and delivery or in further development of our products.
Direct Costs of Revenue. Direct costs of revenue increased by $200,113 compared to the year ended December 31, 2020 principally due to increases in payroll and related expenses.
General and Administrative Expenses. General and administrative expenses increased by $204,445 compared to the year ended December 31, 2020 principally due to increases in consulting expense, payroll and related expenses and professional fees.
Other Income and Expense. During the year ended December 31, 2021 we recognized $103,900 of other income for forgiveness of various PPP loans. We had no such transaction during the year ended December 31, 2020.
Loss from Operations. Our operating loss increased by $463,316 for the year ended December 31, 2021, when compared to a loss of $522,322 for the same period a year ago. The increase was due to the increases in our general and administrative expenses and direct costs of revenue.
Net Loss. Our net loss was $845,843 for the year ended December 31, 2021, as compared to a net loss of $606,451 for the year ended December 31, 2020. The $239,392 increase in net loss was principally due to the increases in our general and administrative expenses and direct costs of revenue.
Liquidity, Capital Resources and Acquisition
At June 30, 2022. At June 30, 2022, we had $6,598 in cash on hand, a working capital deficit of $3,451,460 and an accumulated deficit of approximately $18.6 million. In addition, we incurred a loss from operations of $585,503 and $336,648 for the six months ended June 30, 2022 and 2021, respectively. For the six months ended June 30, 2022 and 2021, we financed our operations with non-interest bearing loans principally from our former parent company.
As of July 1, 2022, the outstanding loan from Rennova to the Company totaled $803,416. The Company signed a promissory note, dated July 1, 2022, in favor of Rennova that provided that the Company will pay Rennova $883,757 on December 31, 2022. That amount represented a 10% original issue discount above the loan amount outstanding on July 1, 2022.
InnovaQor acquired all of the common stock of the HTS Group from Rennova on June 25, 2021. The transaction has been accounted for as a reverse acquisition in the accompanying financial statements.
At December 31, 2021. At December 31, 2021, we had $46 in cash on hand, a working capital deficit of $2,860,200 and an accumulated deficit of approximately $18.0 million. In addition, we incurred a loss from operations of $985,638 and $522,322 for the years ended December 31, 2021 and 2020, respectively. For the years ended December 31, 2021 and 2020, we financed our operations with non-interest bearing loans principally from our former parent company.
| 34 |
InnovaQor acquired all of the common stock of the HTS Group from Rennova on June 25, 2021. The transaction has been accounted for as a reverse acquisition in the accompanying financial statements.
A summary of the Statement of Operations as if the acquisition of the HTS Group had taken place on July 1, 2020 is not presented because it is not materially different than the actual statement of operations.
The change in cash used in operations for the years ended December 31, 2021 and 2020 is presented in the following table:
| Year Ended December 31, | ||||||||||||
| 2021 | 2020 | Change | ||||||||||
| Net loss | $ | (845,843 | ) | $ | (606,451 | ) | $ | -239,392 | ||||
| Non-cash adjustments to loss | ||||||||||||
| Accounts receivable | 118,563 | 331,109 | -212,546 | |||||||||
| Accounts payable and accrued expenses | 349,409 | (121,255 | ) | -470,664 | ||||||||
| Other | (100,998 | ) | 12,131 | -113,129 | ||||||||
| Net cash used in operating activities | $ | (478,869 | ) | $ | (384,466 | ) | $ | -94,403 | ||||
No cash was provided by investing activities for the years ended December 31, 2021 or 2020.
Net cash provided by financing activities amounted to $447,575 and $399,093 for the years ended December 31, 2021 and 2020, respectively. The principal financing activities were loans from the former parent.
Going Concern and Liquidity. Under Accounting Standards Update (“ASU”), 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40), management of InnovaQor has the responsibility to evaluate whether conditions and/or events raise substantial doubt about its ability to meet its future financial obligations as they become due within one year after the date that the financial statements are issued. As required by Accounting Standard Codification (“ASC”) 205-40, this evaluation shall initially not take into consideration the potential mitigating effects of plans that have not been fully implemented as of the date the financial statements are issued. Management has assessed InnovaQor’s ability to continue as a going concern in accordance with the requirement of ASC 205-40.
The Company has incurred net losses of $18,616,660 since inception, raising substantial doubt about its ability to continue as a going concern. Management believes that the Company’s ability to continue as a going concern is dependent on its ability to raise capital, generate revenue, achieve profitable operations and repay its obligations when they come due. As of June 30, 2022, we had $6,598 in cash and we owed $12,802,660 for various liabilities. We are pursuing various financing alternatives to address the payment of outstanding liabilities and to support sales, as well as the completion of the elements of our business plan. There can be no assurance, however, that we will obtain adequate funding or that we will be successful in accomplishing any of our objectives. Consequently, we may not be able to continue as an operating company.
InnovaQor’s consolidated financial statements are prepared assuming that InnovaQor can continue as a going concern, which contemplates continuity of operations through realization of assets, and the settling of liabilities in the normal course of business. Management’s plans with respect to alleviating the adverse financial conditions that caused management to express substantial doubt about InnovaQor’s ability to continue as a going concern are as follows:
During 2021, InnovaQor’s Board of Directors approved plans to acquire the HTS Group. Completion of the acquisition occurred on June 25, 2021. The intent of the acquisition was to create a revenue generating business in the health technology space focused on its strengths and operational plans. The acquisition of the HTS Group from Rennova is intended, among other things, to: (1) result in improved business and operational decision-making and greater strategic and management focus for each respective business; (2) improve the Company’s ability to attract, retain and incentivize employees; (3) improve access to capital for InnovaQor; and (4) create an equity structure for InnovaQor, resulting in an improved understanding of InnovaQor in the capital and investor markets, and a stronger, more focused investor base for InnovaQor. Management believes that the acquisition will allow InnovaQor to more fully realize its value, and InnovaQor to use its stock as consideration for further acquisitions and enhance the value of its equity-based compensation programs.
In addition, in connection with the acquisition, at the closing Rennova forgave $14,000,000 of loans it had previously made to the subsidiaries. A further $950,000 in debt was forgiven by Rennova in September 2021, meaning all of the loans owed by the acquired subsidiaries have been forgiven. The Company issued 14,000 shares of its Series B-1 Preferred Stock to Rennova in June 2021 and a further 950 shares in September 2021.
| 35 |
Notwithstanding the benefits that are expected to result from the acquisition, InnovaQor has incurred substantial costs in connection with the acquisition and the transition to being a fully reporting public company, which may include accounting, tax, legal and other professional services costs, recruiting and relocation costs associated with hiring key senior management personnel who are new to InnovaQor, tax costs and costs to separate information systems. The cost of performing such functions is anticipated to be more than the amounts reflected in InnovaQor’s historical financial statements, which could cause its losses to increase. Accordingly, InnovaQor will continue to focus on increasing revenues.
There can be no assurance that, through the acquisition of the HTS Group, InnovaQor will be able to achieve its business plan, raise any additional capital or secure the additional financing necessary from Rennova or third parties to implement its current operating plan. The ability of InnovaQor to continue as a going concern is dependent upon its ability to significantly increase its revenues and eventually achieve profitable operations. The accompanying consolidated financial statements do not include any adjustments that might be necessary if InnovaQor is unable to continue as a going concern.
Management believes the Company’s funds are insufficient to provide for its proposed needs for the next 12 months. The Company is currently working to close additional funding. The Company may have to rely on equity or debt financing that may involve substantial dilution to our then existing stockholders. If it is unable to close additional financing, the Company may have to cease operations.
Capitalization. The following table sets forth InnovaQor’s capitalization as of June 30, 2022 and December 31, 2021, on an historical basis. In addition, it is not indicative of our future capitalization. This table should be read in conjunction with InnovaQor’s financial statements and notes thereto included elsewhere in this registration statement.
The following table sets forth our cash and capitalization as of June 30, 2022 and December 31, 2021:
| June 30, 2022 | December 31, 2021 | |||||||
| Cash | $ | 6,589 | $ | 46 | ||||
| Stockholders’ Equity | ||||||||
| Preferred Series A-1 Stock, Par Value $0.0001, 1,000 shares authorized, 1,000 shares issued and outstanding | — | — | ||||||
| Common Stock, Par Value $0.0001, 325,000,000 shares authorized, 234,953,286 shares issued and outstanding | 23,495 | 23,495 | ||||||
| Additional Paid-in Capital | 5,857,658 | 5,857,658 | ||||||
| Total capitalization | $ | 5,887,742 | $ | 5,881,199 | ||||
Inflation
We do not believe inflation has a significant effect on InnovaQor’s operations.
Off-Balance Sheet Arrangements
Under SEC regulations, we are required to disclose InnovaQor’s off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on InnovaQor’s financial condition, results of operations, liquidity, capital expenditures or capital resources that are material to investors. Off-balance sheet arrangements consist of transactions, agreements, or contractual arrangements to which any entity that is not consolidated with us is a party, under which we have:
| ● | Any obligation under certain guarantee contracts. | |
| ● | Any retained or contingent interest in assets transferred to an unconsolidated entity or similar arrangement that serves as credit, liquidity or market risk support to that entity for such assets. | |
| ● | Any obligation under a contract that would be accounted for as a derivative instrument, except that it is both indexed to InnovaQor’s stock and classified in equity in InnovaQor’s statement of financial position. | |
| ● | Any obligation arising out of a material variable interest held by us in an unconsolidated entity that provides financing, liquidity, market risk or credit risk support to us, or engages in leasing, hedging or research and development services with us. |
As of December 31, 2021, InnovaQor had no off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on InnovaQor’s financial condition, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Recently Issued Accounting Pronouncements
We do not expect the adoption of any recently issued accounting pronouncements to have a significant impact on our net results of operations, financial position, or cash flows.
| 36 |
Seasonality
We do not expect our net revenues to be impacted by seasonal demands for our products and services.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors and Executive Officers
The following table sets forth the names and ages of our company’s current directors and executive officers.
| Name | Age | Position(s) | ||||
Justin Doherty Sharon Hollis Thomas J. Bellante Gerard Dab |
55 46 74 74 |
Director and Chairman Director, President and Chief Executive Officer Acting Chief Financial Officer Director and Secretary |
Our Directors serve until the earlier occurrence of the election of his successor at the next meeting of shareholders, death, resignation or removal by the Board of Directors. Officers serve at the discretion of our Board of Directors. There are no family relationships between or among our directors and executive officers.
Certain information regarding the backgrounds of each of our officers and directors is set forth below.
Justin Doherty is a member of our Board and was appointed to our Board and as Chairman on July 6, 2021. Mr. Doherty brings over 20 years of international experience in integrated facility management and software solutions for multiple industries across the UK, Europe and the USA. Mr. Doherty previously owned and operated Axis Control Systems for over 10 years, where he started, grew and eventually sold the business. His main area of expertise is operational management and process improvement within the technological and healthcare field, with experience ranging from security systems to healthcare solutions and dementia home health care facilities.
Mr. Doherty was until the end of 2018 CEO of Medical Mime and ClinLab from April 2016 and March 2014, respectively, each an InnovaQor company specializing in healthcare technologies for medical practices throughout the United States. He is currently a Board member for Pathlogic Limited. Mr. Doherty is the managing director of Zest Fire and Security Ltd., which he founded in August 2020.
Sharon Hollis is a member of our Board and was appointed to our Board and as our Chief Executive Officer on July 6, 2021. Ms. Hollis transitioned from property sales in New York and Florida into healthcare in 2010 when she oversaw the development of a proprietary lab ordering and reporting software, Advantage, which allowed doctors and clinical laboratories to evolve from paper orders and reports with minimal disruption and cost. Ms. Hollis oversaw the evolution of Advantage to a fully integrated product that allowed real time tracking of lab samples and their results from sample collection to reporting and billing. Ms. Hollis sold her interests in this software into the predecessor of Rennova, Medytox Solutions, Inc., in 2012 and was the VP of Operations of the corporate office to 2015 overseeing sales development, customer care, process improvement and development of software products and their integration and medical billing related processes. From October 2015, as the owner of PetVetCorp, Inc., Ms. Hollis built a veterinarian health records software. Ms. Hollis re-joined Rennova in 2016 and became the COO of the software division, HTS in 2018. Ms. Hollis has been focused on setting direction for the software division in anticipation of a separation from Rennova and has been engaged in the provision of software solutions to Rennova’s hospital entities for real-time reporting and analysis of billing related data in easy to utilize dashboards for management. Ms. Hollis’ focus is on the provision of easy-to-use software solutions that create efficient solutions and visual interpretation of necessary and actionable data and information.
Thomas J. Bellante has served as our Acting Chief Financial Officer since February 2022. Mr. Bellante has been practicing in public accounting since 1969. In November 2019, he started the CPA Firm of Thomas J. Bellante CPA PA, where he is the Managing Partner. It is a firm that assists smaller Public Companies with their SEC filing requirements. From 2012 until 2020, Mr. Bellante was the Chief Financial Officer of Garyn Angel Enterprises, Inc., a company that designs, develops, markets and distributes products that provide consumers the ability to refine herbs into topical preparations and ingredients for edibles. He also has been with Surety Accounting Services since their inception in June of 2018. He was a shareholder with that firm handling tax planning, tax return preparation, financial consulting and bookkeeping for its clients. He joined the firm of Pender McNulty & Newkirk in April of 1976. In 1981, he became a partner of that firm. Bellante led that firm’s Audit Department and established the SEC Practice Division. Under his leadership, that firm’s SEC Practice Division was ranked 48th in Bowman First Alert’s 2006 list of the Top 100 Public Company Accounting Firms in the U.S. He served as that firm’s Managing Partner from 1989 to 2005, growing the company to a 52-person CPA firm. In January 2013, Pender Newkirk & Company joined forces with Warren Averett, LLC. Warren Averett, with more than 800 employees, is presently ranked among the nation’s Top 30 accounting firms. Bellante served as a leader of that firm’s SEC Practice Group. Mr. Bellante’s industry experience includes reporting public shell corporations, construction firms, software developers, manufacturing companies, R.V. dealerships, mortgage brokers and bankers, brokerage dealers, international communication system companies, real estate developers, data processing companies, import/export companies, development stage enterprises and multi-state/international corporate conglomerates.
| 37 |
Gerard Dab is a member of our Board and was appointed as a Director, CEO, Chairman of the Board, and Corporate Secretary on October 6, 2004. As part of the agreement with Rennova he resigned his position as CEO and Chairman of the Board on July 6, 2021 and retained the positions of Director and Corporate Secretary. Mr. Dab is a pioneer of the modern healthcare informatics industry – He is notably the co-founder of one of the world’s most innovative systems for the automation of clinical workflow in hospitals and medical facilities that had been originally developed at McGill University Royal Victoria Hospital.
His companies provided advanced software platforms that power clinical applications at point of care in hospitals and clinics developed at a cost of some $50,000,000. These applications offered advanced patient management clinical systems with full diagnostic and medical content including decision support that have been developed by senior McGill University practitioners and were used by more than 1,000 clinicians and care givers in the US and Canada.
Prior to his work in the healthcare field, Mr. Dab worked for advertising giant Foote, Cone & Belding of Chicago following his graduation from McGill University. During the 1980s, he founded and was the managing partner of Productions Publi-Cité, a film and television finance company that helped raise some $100,000,000 in production money for independent film producers. During the 1990s, Dab Communications was noted for producing weekly information programs on Canadian network television about investing, created with the support of Maryland Public Television and Louis Rukeyser, long-time host of Wall Street Week.
Conflicts of Interest
Certain of the officers and directors of the Company are engaged in other businesses, either individually or through partnerships and corporations in which they have an interest, hold an office, or serve on a board of directors. As a result, certain conflicts of interest may arise between the Company and its officers and directors. The Company will attempt to resolve such conflicts of interest in favor of the Company. The officers and directors of the Company are accountable to it and its shareholders as fiduciaries, which require that such officers and directors exercise good faith and integrity in handling the Company’s affairs. A shareholder may be able to institute legal action on behalf of the Company or on behalf of itself and other similarly situated shareholders to recover damages or for other relief in cases of the resolution of conflicts is in any manner prejudicial to the Company.
Corporate Governance
We do not have a separate Compensation Committee, Audit Committee or Nominating Committee. These functions are conducted by our Board of Directors acting as a whole.
There are no understandings between any director of our company or any other person pursuant to which any officer or director was or is to be selected as an officer or director.
Independence of Board of Directors
None of our directors is independent, within the meaning of definitions established by the SEC or any self-regulatory organization. We are not currently subject to any law, rule or regulation requiring that all or any portion of our Board of Directors include independent directors.
Shareholder Communications with Our Board of Directors
Our company welcomes comments and questions from our shareholders. Shareholders should direct all communications to our President and Chief Executive Officer, Sharon Hollis, at our executive offices. However, while we appreciate all comments from shareholders, we may not be able to respond individually to all communications. We will attempt to address shareholder questions and concerns in our press releases and documents filed with the SEC, so that all shareholders have access to information about us at the same time. Ms. Hollis collects and evaluates all shareholder communications. All communications addressed to our directors and executive officers will be reviewed by those parties, unless the communication is clearly frivolous.
Code of Ethics
As of the date of this Offering Circular, our Board of Directors has not adopted a code of ethics with respect to our directors, officers and employees. As our securities are not listed on a national securities exchange, we are not required and do not have a written code of business conduct and ethics that applies to our directors, officers and employees. Our management does, however, promote honest and ethical conduct, full and fair disclosure in our reports to the SEC, and compliance with applicable governmental laws and regulations.
| 38 |
In General
Currently, our management is unable to estimate accurately when, if ever, our company will possess sufficient capital, whether derived from sales revenues, this offering or otherwise, for the payment of salaries to our management.
As of the date of this Offering Circular, there are no annuity, pension or retirement benefits proposed to be paid to officers, directors or employees of our company, pursuant to any presently existing plan provided by, or contributed to, our company.
Compensation Summary
The Board of Directors has approved a compensation for its CEO, Sharon L. Hollis of $250,000 a year and approved compensation of $7,500 a month for Thomas J. Bellante to act as a part time contracted CFO.
Currently, there are no other executives but InnovaQor plans to appoint additional executives and may reimburse its executives for expenses incurred in connection with travel, networking, and day to day business development.
The following table sets forth all of the compensation awarded to, earned by or paid to each individual that served as our principal executive officer or principal financial officer during the years ended December 31, 2021 and 2020. The Company did not have any other executive officers during the years ended December 31, 2021 and 2020. Prior to July 6, 2021, Mr. Dab performed the duties of both the principal executive officer and the principal financial officer.
The following table summarizes information concerning the compensation awarded, paid to or earned by, our executive officers.
| Name and Principal Position | Fiscal Year Ended 6/30 | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Non-qualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||||||||
| Sharon Hollis(1) | 2021 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| President, CEO | 2020 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Gerard Dab(2) | 2021 | — | — | — | — | — | — | 500,000 | (3) | 500,000 | (3) | |||||||||||||||||||||||||
Secretary, Former Chief Executive Officer | 2020 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
(1) |
Ms. Hollis did not become an officer of our company until June 2021. |
| (2) | Mr. Dab resigned as our Chief Executive Officer on July 6, 2021. He continues to act as Secretary and as a Director. |
| (3) | Pursuant to an agreement with our company, Mr. Dab provided services in connection with the acquisition of the Group in exchange for $500,000. Mr. Dab then forgave $300,000 owed to him by our company in exchange for 1,000 shares of Series A-1 Supermajority Voting Preferred Stock and forgave an additional $200,000 owed to him by our company in exchange for 200 shares of Series C-1 Convertible Redeemable Preferred Stock. |
Outstanding Option Awards
The following table provides certain information regarding unexercised options to purchase common stock, stock options that have not vested and equity-incentive plan awards outstanding as of the date of this Offering Circular, for each named executive officer.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) | |||||||||||||||||||||||||||
| Sharon Hollis | — | — | — | — | n/a | — | n/a | — | — | |||||||||||||||||||||||||||
| Gerard Dab | — | — | — | — | n/a | — | n/a | — | — | |||||||||||||||||||||||||||
| 39 |
Agreements with Named Executive Officers
Gerard Dab. We entered into a consulting agreement effective June 1, 2021, with a company owned by Mr. Dab for a period of one year to assist in developing our business, including communications with existing shareholders and the general public. This company shall be paid $60,000 upon receipt of funding from an outside source or within 90 days of signing the agreement. This has not yet been paid. Additionally, this company shall be paid $3,500 per month until the agreement expires.
Outstanding Equity Awards
Our Board of Directors has made no equity awards and no such award is pending.
Long-Term Incentive Plans
We currently have no employee incentive plans.
Director Compensation
InnovaQor has not paid any director’s fees or other cash compensation for services rendered as a director since the acquisition of the HTS Group to the date of this filing. Compensation of $30,000 per year for each director (excluding the CEO) has been agreed for the next 12 months.
We do not pay employee directors for Board service in addition to their regular employee compensation. The Board has the primary responsibility for considering and determining the amount of director compensation.
The following table shows amounts earned by each non-employee Director in the years ended December 31, 2021 and 2020:
Director | Fees earned or paid in cash | Stock Awards | Option Awards | Non-equity Incentive Plan Compensation | All Other Compensation | Total | ||||||||||||||||||
| Gerard Dab | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Lewis Lombardo (1) | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Justin Doherty (2) | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — |
| (1) | Mr. Lombardo resigned as a Director in connection with the acquisition of the Group on June 25, 2021. |
| (2) | Mr. Doherty was elected to the Board of Directors on July 6, 2021. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The table below does not give effect to the following:
Series B-1 Convertible Redeemable Preferred Stock Conversion. The table below does not give effect to the issuance of shares of our common stock upon conversion of the outstanding shares of Series B-1 Convertible Redeemable Preferred Stock, 99.33% of which is owned by Rennova Health, Inc. (see Note 10 in the table below). Until September 2022, no shares of our outstanding Series B-1 Convertible Redeemable Preferred Stock may be converted, without the consent of Epizon Limited, as owner of all of our outstanding Series A-1 Supermajority Voting Preferred Stock, while no such consent is required thereafter; provided, however, that no conversion of Series B-1 Convertible Redeemable Preferred Stock can be made to the extent the converting holder’s beneficial interest in our common stock exceeds 4.99%. Notwithstanding such limitations, were all outstanding shares of our Series B-1 Convertible Redeemable Preferred Stock be eligible for conversion, we would be obligated to issue approximately 3,800,000,000 shares of common stock, based on recent market prices for our common stock. (See “Risk Factors—Risks Related to a Purchase of the Offered Shares” and “Dilution—Ownership Dilution”).
Series C-1 Convertible Redeemable Preferred Stock Conversion. The table below does not give effect to the issuance of shares of our common stock upon conversion of the outstanding shares of Series C-1 Convertible Redeemable Preferred Stock, all of which are owned by our officers and directors. Until the date that his one year from their issuance, no shares of our outstanding Series C-1 Convertible Redeemable Preferred Stock may be converted, without the consent of Epizon Limited, as owner of all of our outstanding Series A-1 Supermajority Voting Preferred Stock, while no such consent is required thereafter; provided, however, that no conversion of Series C-1 Convertible Redeemable Preferred Stock can be made to the extent the converting holder’s beneficial interest in our common stock exceeds 4.99%. Notwithstanding such limitations, were all outstanding shares of our Series C-1 Convertible Redeemable Preferred Stock be eligible for conversion, we would be obligated to issue approximately 50,000,000 shares of common stock, based on recent market prices for our common stock. (See “Risk Factors—Risks Related to a Purchase of the Offered Shares” and “Dilution—Ownership Dilution”).
| 40 |
In light of the caveats set forth above, as of the date of this Offering Circular, we had 234,953,286 shares of common stock issued and outstanding. The following table sets forth information known to us relating to the beneficial ownership of shares of our voting securities by: each person who is known by us to be the beneficial owner of more than 5% of our outstanding voting stock; each director; each named executive officer; and all named executive officers and directors as a group. The percentages in the table have been calculated on the basis of treating as outstanding for a particular person, all shares of our common stock outstanding on that date and all shares of our common stock issuable to that holder in the event of exercise of outstanding options, warrants, rights or conversion privileges owned by that person at that date which are exercisable within 60 days of that date. Except as otherwise indicated, the persons listed below have sole voting and investment power with respect to all shares of our common stock owned by them, except to the extent that power may be shared with a spouse. The address for each of our executive officers and directors is c/o InnovaQor, Inc., 400 South Australian Avenue, Suite 800, West Palm Beach, Florida 33401.
Share Ownership Before This Offering |
Share Ownership After This Offering |
|||||||||||||||||
| Name of Shareholder | Number of Shares Beneficially Owned |
% Beneficially Owned(1) |
Number of Shares Beneficially Owned |
% Beneficially Owned(2) |
Effective Voting Power | |||||||||||||
| Common Stock | ||||||||||||||||||
| Executive Officers and Directors | ||||||||||||||||||
| Justin Doherty | 0 | 0 | % | 0 | 0 | % | See Note 3, | |||||||||||
| Sharon Hollis | 0 | 0 | % | 0 | 0 | % | Note 4 and Note 5 | |||||||||||
| Thomas J. Bellante | 0 | 0 | % | 0 | 0 | % | ||||||||||||
| Gerard Dab | 0 | 0 | % | 0 | 0 | % | ||||||||||||
| Officers and directors, as a group (4 persons) | 0 | 0 | % | 0 | 0 | % | ||||||||||||
| 5% Owners | ||||||||||||||||||
| Michel Dab(6) | 27,785,000 | 11.82 | % | 27,785,000 | 9.96 | % | ||||||||||||
| Ithaca Scientific Ventures LLC(7) | 22,000,000 | 9.36 | % | 22,000,000 | 7.10 | % | ||||||||||||
| Real Gauthier(8) | 18,300,000 | 7.79 | % | 18,300,000 | 5.91 | % | ||||||||||||
Series A-1 Supermajority Voting Preferred Stock(4) |
||||||||||||||||||
| Epizon Limited(5) | 1,000 | 100 | % | 1,000 | 100 | % | ||||||||||||
Series B-1 Convertible Redeemable Preferred Stock(9) |
||||||||||||||||||
| Rennova Health, Inc.(10) | 14,850 | 99.33 | % | 14,850 | 99.33 | % | ||||||||||||
| William D’Anieri | 100 | 0.67 | % | 100 | 0.67 | % | ||||||||||||
Series C-1 Convertible Redeemable Preferred Stock(11) |
||||||||||||||||||
| Gerard Dab | 200 | 88.89 | % | 200 | 88.89 | % | ||||||||||||
| Sharon Hollis | 25 | 11.11 | % | 25 | 11.11 | % | ||||||||||||
| (1) | Based on 234,953,286 shares outstanding, before this offering. |
| (2) | Based on 309,953,286 shares outstanding, after this offering. |
| (3) | Epizon Limited owns all of the outstanding shares of Series A-1 Supermajority Voting Preferred Stock. By such ownership, Epizon Limited controls the management and affairs of our company, as well as matters requiring the approval by our shareholders, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets, and any other significant corporate transaction. (See Note 4 and Note 5). |
| (4) | The shares of Series A-1 Supermajority Voting Preferred Stock have the following voting rights: So long as one share of Series A-1 Supermajority Voting Preferred Stock is outstanding, the outstanding shares of the Series A-1 Supermajority Voting Preferred Stock shall have the number of votes, in the aggregate, equal to 51% of all votes entitled to be voted at any shareholder meeting. (See “Description of Securities—Preferred Stock—Series A-1 Supermajority Voting Preferred Stock”). |
| (5) | Epizon Limited’s address is Suite 104a, Saffrey Square, Bank Lane, P.O. Box N-9306, Nassau, Bahamas. All of the outstanding capital stock of Epizon Limited is owned by The Shanoven Trust, of which P. Wilhelm F. Tooth serves as trustee. Seamus Lagan is the settlor and Mr. Lagan’s family are beneficiaries of The Shanoven Trust. Mr. Lagan is also the Chief Executive Officer of Rennova Health, Inc. (See Note 10). |
| (6) | Michel Dab is Gerard Dab’s adult son. Michael Dab was our Treasurer from December 15, 2008, until June 29, 2021. Mr. Dab’s address is 50 High Park Avenue, Apt.1609, Toronto, Ontario, Canada M6P 2R9. |
| (7) | The address of this entity is 30 N. Gould Street, Suite R, Sheridan, Wyoming 82801. Dr. Linda McHarg is the sole owner and control person of Ithaca Scientific Ventures. Dr. McHarg is married to Gerard Dab. Under Quebec law, Mr. Dab and Dr. McHarg are subject to the matrimonial regime of separation as to property. Each spouse remains the owner of his or her property and administers the property alone. As a result, Mr. Dab disclaims any beneficial interest in any securities owned by Ithaca Scientific Ventures or Dr. McHarg and Dr. McHarg similarly disclaims any beneficial interest in any securities owned by Mr. Dab. |
| (8) | Mr. Gauthier’s address is 225 Chabanel, Suite 114, Montreal, Quebec, Canada H2N 2C9. |
| 41 |
| (9) | The Series B-1 Convertible Redeemable Preferred Stock has a stated value of $1,000. Each share of Series B-1 Convertible Redeemable Preferred Stock is convertible into shares of our common stock based on the stated value per share at a conversion price equal to 90% of the average closing price of our common stock for the ten trading days immediately prior to the conversion date. The Series B-1 Convertible Redeemable Preferred Stock may not be converted prior to the first anniversary of issuance, except with the consent of the holders of a majority of the then-outstanding shares, if any, of the Series A-1 Supermajority Voting Preferred Stock. No conversion can be made to the extent the holder’s beneficial interest in our common stock exceeds 4.99%. Each share of Series A-1 Supermajority Voting Preferred Stock is redeemable at our company’s option at its stated value, $1,000, plus declared and unpaid dividends. (See “Dilution—Ownership Dilution”). |
| (10) | Seamus Lagan is the Chief Executive Officer of this entity. Rennova Health, Inc.’s address is 400 South Australian Avenue, Suite 800, West Palm Beach, Florida 33401. |
| (11) | The Series C-1 Convertible Redeemable Preferred Stock has a stated value of $1,000. Each share of Series C-1 Convertible Redeemable Preferred Stock is convertible into shares of our common stock based on the stated value per share at a conversion price equal to 90% of the average closing price of our common stock for the ten trading days immediately prior to the conversion date. The Series C-1 Convertible Redeemable Preferred Stock may not be converted prior to the first anniversary of issuance, except with the consent of the holders of a majority of the then-outstanding shares, if any, of the Series A-1 Supermajority Voting Preferred Stock. No conversion can be made to the extent the holder’s beneficial interest in our common stock exceeds 4.99%. Each share of Series A-1 Supermajority Voting Preferred Stock is redeemable at our company’s option at its stated value, $1,000, plus declared and unpaid dividends. (See “Dilution—Ownership Dilution”). |
Series A-1 Supermajority Voting Preferred Stock
We have authorized and outstanding 1,000 shares (stated value $10) of Series A-1 Supermajority Voting Preferred Stock, as of the date of this Offering Circular. All of our Series A-1 Supermajority Voting Preferred Stock is owned by Epizon Limited, who, through such ownership, controls all corporate matters of our company.
The Series A-1 Supermajority Voting Preferred Stock has the following voting rights: so long as one share of Series A-1 Supermajority Voting Preferred Stock is outstanding, the outstanding shares of the Series A-1 Supermajority Voting Preferred Stock shall have the number of votes, in the aggregate, equal to 51% of all votes entitled to be voted at any shareholder meeting. Epizon Limited will, therefore, be able to control the management and affairs of our company, as well as matters requiring the approval by our shareholders, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets, and any other significant corporate transaction. (See “Risk Factors—Risks Related to a Purchase of the Offered Shares” and “Description of Securities—Preferred Stock—Series A-1 Supermajority Voting Preferred Stock”).
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Acquisitions and Change in Control
In late 2020, the majority of shareholders and the Board of Directors charged management of VisualMED to find a new business opportunity for the Company. At the beginning of 2021, the Company initiated measures that would facilitate a new opportunity for the Company. Subsequently, in May 2021, then CEO Gerard Dab and VisualMED entered into an agreement with and engaged the services of Epizon Limited (“Epizon”), a Nassau, Bahamas, based management consulting company specializing in the provision of management services to secure financing and opportunities for growth. Seamus Lagan, the Chief Executive Officer of Rennova Health, Inc., the company we ultimately completed a transaction with is also the managing director of Epizon Limited.
The objective of the agreement with Epizon was to help VisualMED find a new opportunity in its core healthcare technology business. The Company needed to find and develop new products that would be more relevant for a changing healthcare marketplace. Epizon was engaged to assist VisualMED with its capital structure, and to look for new business opportunities and/or acquisitions that could result in improved shareholder value. The terms of the agreement with Epizon called for the transfer to Epizon of 1,000 shares of Series A-1 Supermajority Voting Preferred Stock, with a stated value of $10.00 each, personally owned by Gerard Dab, on the successful completion of a transaction as defined in the agreement. It was determined that an agreement with Rennova Health, Inc. as defined was the most viable opportunity available to VisualMED. The conditions of the Epizon agreement have been met and the transfer of shares of Series A-1 Supermajority Voting Preferred Stock has been completed. This transfer resulted in a change of voting control of VisualMED, as the Series A-1 Supermajority Voting Preferred Stock, in the aggregate, has the right to the number of votes equal to 51% of the votes entitled to be cast at a meeting or to vote by written consent. There is no material value attached to the shares of Series A-1 Supermajority Voting Preferred Stock and no arrangement or agreement that could result in Epizon receiving financial consideration for completing the transaction as defined, with Rennova Health, Inc.
In May 2021, VisualMED entered into an acquisition agreement with Rennova to acquire certain assets owned by Rennova.
On June 25, 2021, VisualMED closed the acquisition agreement with Rennova , and acquired 100% ownership of certain subsidiaries of Rennova. These subsidiaries are Health Technology Solutions, Inc., Medical Mime, Inc., ClinLab, Inc., Advanced Molecular Services Group, Inc., Genomas, Inc. and CollabRx, Inc., and combined are referred to herein as HTS and AMSG (the “HTS Group”).
Products offered by the acquired entities include vCIO services, IT managed services, healthcare finance and operational business intelligence analytics dashboards, an her (electronic health records software), an LIS (laboratory information system), a lab ordering and reporting software, actionable data analytics and reporting for oncologists to enhance cancer diagnoses and treatment and PhyzioType Systems for DNA-guided management and prescription of drugs.
The Company intends to operate its subsidiaries under the following structure.
In consideration for the shares of HTS and AMSG and the elimination of inter-company debt between Rennova and HTS and AMSG, the Company issued 14,000 shares of its Series B-1 Convertible Redeemable Preferred Stock to Rennova. The number of shares of Series B-1 Convertible Redeemable Preferred Stock was subject to a post-closing adjustment which resulted in 950 additional shares of Series B-1 Convertible Redeemable Preferred Stock due Rennova which were issued in September 2021. Each share of Series B-1 Convertible Redeemable Preferred Stock has a stated value of $1,000 and is convertible into that number of shares of the Company’s common stock equal to the product of the stated value divided by 90% of the average closing price of the common stock during the 10 trading days immediately prior to the conversion date. Conversion of the Series B-1 Convertible Redeemable Preferred Stock, however, is subject to the limitation that no conversion can be made to the extent the holder’s beneficial interest (as defined pursuant to the terms of the Series B-1 Convertible Redeemable Preferred Stock) in the common stock of the Company would exceed 4.99%. The shares of Series B-1 Convertible Redeemable Preferred Stock may be redeemed by the Company upon payment of the stated value of the shares plus any accrued declared and unpaid dividends.
| 42 |
In addition, prior to the acquisition transactions, the Company’s former CEO, Gerard Dab, forgave $300,000 owed to him by the Company in exchange for the issuance of 1,000 shares of Series A-1 Supermajority Voting Preferred Stock. These shares of Series A-1 Supermajority Voting Preferred Stock have now been transferred to Epizon. The former CEO of the Company also forgave another $200,000 owed to him from the Company in exchange for 200 shares of Series C-1 Convertible Redeemable Preferred Stock with each share having a stated value of $1,000 and convertible into that number of shares of common stock equal to the product of the stated value divided by 90% of the average closing price of the previous 10 trading days immediately prior to the conversion date. Conversion of the Series C-1 Convertible Redeemable Preferred Stock is also subject to a similar 4.99% beneficial ownership limitation. Shares of the Series B-1 Convertible Redeemable Preferred Stock and Series C-1 Convertible Redeemable Preferred Stock may not be converted prior to the first anniversary of their issuance without the consent of the holders of a majority of the then outstanding shares, if any, of the Series A-1 Supermajority Voting Preferred Stock.
Certain Transactions with Former CEO
The former CEO of our company, Gerard Dab, paid all the disbursements on our behalf for all periods presented. The cash disbursements amounted to $11,385 and $20,388 for the years ended June 30, 2021 and 2020, respectively, and are shown in the accompanying Statements of Operations as operating expenses.
In addition, for the year ended June 30, 2021, we incurred $500,000 for services of Mr. Dab in connection with the acquisition of the Group, which is shown in the accompanying Statements of Operations as a general and administrative expense.
The above amounts are not necessarily indictive of that to which third parties would have agreed.
Loans from Related Parties
Mr. Dab, the former CEO of InnovaQor, paid certain of the disbursements on behalf of the Company for the years ended December 31, 2021 and 2020. The cash disbursements amounted to $24,993 and $7,670 for the years ended December 31, 2021 and 2020, respectively, and are included in the accompanying Statements of Operations as operating expenses.
In addition, during the year ended December 31, 2021 under an agreement with the Company, Mr. Dab provided services in connection with the acquisition of the Group in exchange for $500,000. This was reflected in the accompanying Statement of Operations as a General and Administrative Expense. Mr. Dab then forgave the $500,000 owed to him by the Company in exchange for shares of Series A-1 Preferred Stock and Series C-1 Preferred Stock as described above.
During the six months ended June 30, 2022, Ms. Hollis, the Chief Executive Officer of the Company, loaned the Company $90,100. The Company entered into a promissory note in the amount of $98,510, representing a 10% original issue discount. The loan is due on September 6, 2022; provided that, 50% is due if the Company receives $500,000 of financing and 100% is due if the Company receives $1,000,000 of financing. In addition, the Company issued Ms. Hollis 25 shares of Series C-1 Preferred Stock on March 31, 2022 in connection with the loan. Ms. Hollis, subsequent to June 30, 2022, has advanced a further $6,000 to the Company, which is repyable on demand.
During the three and six months ended June 30, 2022, the Company contracted with Rennova to provide Rennova ongoing health information technology-related services totaling approximately $50,000 and $0.1 million, respectively. In addition, the Company currently subleases office space from Rennova on a month-to-month term at a cost of approximately $9,700 per month for rent and utilities.
As of July 1, 2022, the Company owed Rennova $803,416 from advances made by Rennova. The Company signed a promissory note in favor of Rennova that provides that the Company will repay Rennova $883,757 on December 31, 2022. That amount represents a 10% original issue discount above the loan amount outstanding on July 1, 2022. The note, in the event of default, bears interest at 18% per annum.
Form July 29, 2022 through September 1, 2022, Alcimede Limited, of which Seamus Lagan is the director, has loaned the Company an aggregate of $32,500. These loans are due on demand. Upon any repayment, the Company will also owe an amount equal to 10% of the amount repaid.
The above amounts are not necessarily indicative of terms to which third parties would have agreed.
Haynie & Company, an independent registered public accounting firm, has audited our financial statements at December 31, 2021 and 2020, as set forth in its report. We have included our financial statements in this Offering Circular in reliance on Haynie & Company’s report, given on their authority as experts in accounting and auditing.
Certain legal matters with respect to the Offered Shares offered by this Offering Circular will be passed upon by Newlan Law Firm, PLLC, Flower Mound, Texas.
WHERE YOU CAN FIND MORE INFORMATION
We have filed an offering statement on Form 1-A with the SEC under the Securities Act with respect to the common stock offered by this Offering Circular. This Offering Circular, which constitutes a part of the offering statement, does not contain all of the information set forth in the offering statement or the exhibits and schedules filed therewith. For further information with respect to us and our common stock, please see the offering statement and the exhibits and schedules filed with the offering statement. Statements contained in this Offering Circular regarding the contents of any contract or any other document that is filed as an exhibit to the offering statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the offering statement. The offering statement, including its exhibits and schedules, may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549, and copies of all or any part of the offering statement may be obtained from such offices upon the payment of the fees prescribed by the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains an Internet website that contains all information regarding companies that file electronically with the SEC. The address of the website is www.sec.gov.
| 43 |
CONSOLIDATED FINANCIAL STATEMENTS
Index to Financial Statements
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of InnovaQor, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of InnovaQor, Inc. (the Company) as of December 31, 2021 and 2020, and the related statements of operations, stockholders’ deficit, and cash flows for each of the years in the two-year period ended December 31, 2021, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has accumulated significant losses and has negative cash flows from operations and, at December 31, 2021, had a $2.9 million working capital deficit and $18 million accumulated deficit. In addition, the Company’s cash position is critically deficient and critical payments are not being made in the ordinary course of business, all of which raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Haynie & Company
Haynie & Company
Salt Lake City, Utah
July 29, 2022
PCAOB #457
We have served as the Company’s auditor since 2021
| F-2 |
CONSOLIDATED BALANCE SHEETS
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 46 | $ | 31,294 | ||||
| Accounts receivable, net (including related party receivable of $21,852 and $135,687 at December 31, 2021 and 2020, respectively) | 32,800 | 151,363 | ||||||
| Prepaid expenses and other current assets | - | 1,717 | ||||||
| Total current assets | 32,846 | 184,374 | ||||||
| Property and equipment, net | — | 685 | ||||||
| Total assets | $ | 32,846 | $ | 185,059 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 937,698 | $ | 726,220 | ||||
| Accrued expenses | 1,446,722 | 1,308,283 | ||||||
| Due to Former Parent | 374,473 | — | ||||||
| Current portion of notes payable | 134,153 | 134,118 | ||||||
| Total current liabilities | 2,893,046 | 2,168,621 | ||||||
| Notes payable – Long term | 60,401 | 103,900 | ||||||
| Preferred Series B-1 Stock, Par Value $0.0001, 25,000 shares authorized, 14,950 and 0 shares issued and outstanding, December 31, 2021 and 2020, respectively | 9,086,396 | — | ||||||
| Preferred Series C-1 Stock, Par Value $0.0001, 2,000 shares authorized, 200 and 0 shares issued and outstanding, December 31, 2021 and 2020, respectively | 122,000 | — | ||||||
| Total liabilities | 12,161,843 | 2,272,521 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ Deficit | ||||||||
| Preferred Series A-1 Stock, Par Value $0.0001, 1,000 shares authorized, 1,000 and 0 shares issued and outstanding, December 31, 2021 and 2020, respectively | — | — | ||||||
| Common Stock, Par Value $0.0001, 325,000,000 shares authorized, 234,953,286 and 0 shares issued and outstanding, December 31, 2021 and 2020, respectively | 23,495 | — | ||||||
| Additional Paid-In Capital | 5,857,658 | — | ||||||
| Accumulated Deficit | (18,010,150 | ) | — | |||||
| Parent’s net investment deficit in Advanced Molecular and Health Technology Solutions Group | — | (2,087,462 | ) | |||||
| Total Stockholders’ Deficit | (12,128,997 | ) | (2,087,462 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 32,846 | $ | 185,059 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
CONSOLIDATED STATEMENTS OF OPERATIONS
| Years Ended | ||||||||
| December 31, 2021 | December 31, 2020 | |||||||
| Net revenues (including net revenue from related party of $237,551 and $185,892 for the years ended December 31, 2021 and 2020, respectively) | $ | 468,883 | $ | 528,624 | ||||
| Operating expenses: | ||||||||
| Direct costs of revenue | 205,649 | 5,536 | ||||||
| General and administrative expenses | 1,247,687 | 1,043,242 | ||||||
| Depreciation | 1,185 | 2,168 | ||||||
| Total operating expenses | 1,454,521 | 1,050,946 | ||||||
| Loss from operations | $ | (985,638 | ) | $ | (522,322 | ) | ||
| Other (expense) income: | ||||||||
| Other (expense) income, net | 104,918 | 24,668 | ||||||
| Interest expense and payroll tax penalties | 34,877 | (108,797 | ) | |||||
| Total other (expense) income | 139,795 | (84,129 | ) | |||||
| Loss before income taxes | (845,843 | ) | (606,451 | ) | ||||
| Provision for income taxes | – | – | ||||||
| Net loss | $ | (845,843 | ) | $ | (606,451 | ) | ||
| Basic and Diluted (loss) per share | $ | (0.00 | ) | |||||
| Basic and Diluted weighted average shares of common stock outstanding | 243,782,416 | |||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
| Preferred Series A-1 - Shares | Preferred Series A-1 - Par Value | Common Stock - Shares | Common Stock – Par Value | Additional Paid-In Capital | Accumulated Deficit | Total | ||||||||||||||||||||||
| Balance at December 31, 2019 | - | - | - | - | $ | 14,659,496 | $ | (16,557,856 | ) | $ | (1,898,360 | ) | ||||||||||||||||
| Borrowings From Former Parent | - | - | - | - | 417,349 | 417,349 | ||||||||||||||||||||||
| Net loss for year | - | - | (606,451 | ) | (606,451 | ) | ||||||||||||||||||||||
| Balance at December 31, 2020 | - | $ | - | - | $ | - | 15,076,845 | (17,164,307 | ) | (2,087,462 | ) | |||||||||||||||||
| Issuance of Stock in Transaction | 1,000 | - | 234,953,286 | 23,495 | (23,495 | ) | - | - | ||||||||||||||||||||
| Issuance of Preferred Stock as part of reverse merger | - | - | - | - | (9,195,692 | ) | - | (9,195,692 | ) | |||||||||||||||||||
| Net loss for year | - | - | - | - | - | (845,843 | ) | (845,843 | ) | |||||||||||||||||||
| Balance at December 31, 2021 | 1,000 | $ | - | 234,953,286 | $ | 23,495 | $ | 5,857,658 | $ | (18,010,150 | ) | $ | (12,128,997 | ) | ||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended | ||||||||
| December 31, 2021 | December 31, 2020 | |||||||
| Cash flows (used in) operating activities: | ||||||||
| Net loss | $ | (845,843 | ) | $ | (606,451 | ) | ||
| Adjustments to reconcile net loss to net cash (used in) operations: | ||||||||
| Forgiveness of PPP Loans | (103,900 | ) | — | |||||
| Depreciation | 1,185 | 2,168 | ||||||
| Write off of Fixed Assets | — | 501 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | 118,563 | 331,109 | ||||||
| Prepaid expenses | 1,717 | 3,433 | ||||||
| Deposits | — | 6,029 | ||||||
| Accounts payable and accrued expenses | 349,409 | (121,255 | ) | |||||
| Net cash (used in) operating activities | (478,869 | ) | (384,466 | ) | ||||
| Cash flows provided by investing activities: | ||||||||
| Cash acquired in acquisition | 46 | — | ||||||
| Net cash provided by investing activities | 46 | — | ||||||
| Cash flows provided by financing activities: | ||||||||
| Loans from Former Parent | 374,473 | 417,349 | ||||||
| Payments on notes payable | — | (122,156 | ) | |||||
| Reverse merger | 12,701 | — | ||||||
| Proceeds from Paycheck Protection Program Loans | 60,401 | 103,900 | ||||||
| Net cash provided by financing activities | 447,575 | 399,093 | ||||||
| Net (Decrease) Increase in cash | (31,248 | ) | 14,627 | |||||
| Cash at beginning of period | 31,294 | 16,667 | ||||||
| Cash at end of period | $ | 46 | $ | 31,294 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 – Description of Business
InnovaQor, Inc. (which changed its name from VisualMED Clinical Solutions Corporation in September 2021) (“InnovaQor” or the “Company”) was incorporated in the State of Nevada on September 7, 1999. Its business plan involved the distribution of medical software. It was primarily involved in activities related to the distribution of medical software through associated companies to which it has granted operating and distribution licenses.
During 2017, Rennova Health, Inc. (“Rennova” or the “Parent”), the Parent of the Advanced Molecular Services Group, Inc. (“AMSG”) and Health Technology Solutions, Inc. (“HTS”) (collectively, the “Advanced Molecular and Health Technology Solutions Group,” or the “Group”), announced its intent to separate the Group into one or more separate public entities with AMSG holding and operating Rennova’s pharmacogenomics business and HTS holding and operating Rennova’s supportive software solutions business. Pharmacogenomics is the genetic process to understand how an individual’s genetic attributes affect the likely response to therapeutic drugs. HTS’s supportive software solutions business includes electronic health records and laboratory information management systems. AMSG was a wholly-owned subsidiary of Rennova that was formed on May 4, 2017 and HTS was a wholly-owned subsidiary of Rennova that was formed on June 22, 2011.
AMSG’s financial results include the assets and operations of CollabRx, Inc. and Genomas, Inc. Genomas, Inc. operated a diagnostics lab until December 31, 2019 and was focused solely on the technology and platform to interpret diagnostics outcomes and translate these outcomes into easily usable information. HTS’s financial results include the assets and operations of two other strategic businesses formerly owned by Rennova: ClinLab, Inc. and Medical Mime, Inc.
On June 25, 2021, Rennova sold all the shares of stock of its subsidiaries, HTS and AMSG, to InnovaQor in a transaction that has been accounted for as a reverse acquisition with the Group being the accounting acquirer.
In consideration for the shares of HTS and AMSG (HTS Group) and the elimination of inter-company debt between Rennova and HTS and AMSG, InnovaQor issued to Rennova 14,000 shares of its Series B-1 Convertible Redeemable Preferred Stock (the “Series B-1 Preferred Stock”). The number of shares of Series B-1 Preferred Stock was subject to a post-closing adjustment which resulted in an additional 950 shares of Series B-1 Preferred Stock due Rennova, which were issued in September 2021. Each share of Series B-1 Preferred Stock has a stated value of $1,000 and is convertible into that number of shares of InnovaQor’s common stock equal to the product of the stated value divided by 90% of the average closing price of InnovaQor’s common stock during the 10 trading days immediately prior to the conversion date. Conversion of the Series B-1 Preferred Stock, however, is subject to the limitation that no conversion can be made to the extent the holder’s beneficial interest (as defined pursuant to the terms of the Series B-1 Preferred Stock) in the common stock of InnovaQor would exceed 4.99%. The fair market value of the 14,950 shares was $9,086,396, as described below. The shares of Series B-1 Preferred Stock may be redeemed by InnovaQor upon payment of the stated value of the shares plus any declared and unpaid dividends. Because these shares are convertible, at the option of the holder, into a variable number of common shares based solely on a fixed dollar amount (stated value) known at issuance of the shares, they have been recorded as a long-term liability at the date of issuance in accordance with ASC 480, Distinguishing Liabilities from Equity.
On June 9, 2021, InnovaQor issued 1,000 shares of Series A-1 Supermajority Voting Preferred Stock (the “Series A-1 Preferred Stock”) to the then CEO of the Company, Mr. Gerard Dab, in exchange for $300,000 owed to Mr. Dab. The Series A-1 Preferred Stock has the right to the number of votes equal to 51% of the votes entitled to be cast at a meeting or to vote by written consent, meaning the owner of the Series A-1 Preferred Stock has voting control of the Company. Mr. Dab was a party to an agreement whereby he committed to transfer the Series A-1 Preferred Stock to Epizon Limited (“Epizon”), a Nassau, Bahamas, based management consulting company. Seamus Lagan, the Chief Executive Officer of Rennova, the company we ultimately completed a transaction with, is also the managing director of Epizon. The conditions of the Epizon agreement to which Mr. Dab was a party were met and the transfer of shares of Series A-1 Preferred Stock to Epizon was completed. The terms of the agreement between Mr. Dab and Epizon had certain conditions including a condition that if within 120 days after a transaction was completed by VisualMED, there were not a dispute or efforts to unwind the transaction, then Mr. Dab would deliver the shares of Series A-1 Preferred Stock owned by him to Epizon. Epizon, as the owner of the Series A-1 Preferred Stock, will be able to exercise control over all matters submitted for stockholder approval.
| F-7 |
InnovaQor issued 200 shares of Series C-1 Convertible Redeemable Preferred Stock (the “Series C-1 Preferred Stock”) to Mr. Dab in exchange for $200,000 owed to him. The shares had a fair market value of $122,000 at the date of issuance, as described below. Because these shares are convertible, at the option of the holder, into a variable number of common shares based solely on a fixed dollar amount (stated value) known at issuance of the shares, they have been recorded as a long-term liability at the date of issuance in accordance with ASC 480, Distinguishing Liabilities from Equity.
The fair market value of all of the above shares of Series B-1 and Series C-1 Preferred Stock is based on the Option Price Method (the “OPM”). The OPM treats common and preferred interests as call options on the equity value of the subject company, with exercise prices based on the liquidation preference of the preferred interests and participation thresholds for subordinated classes. The Black-Scholes model was used to price the call options. The assumptions used were: risk free rate of 0.84%, volatility of 250.0%, and exit period of 5 years. Lastly, a discount rate of 35% was applied due to the lack of marketability of the InnovaQor preferred stock and the underlying liquidity of InnovaQor’s common stock.
Additionally, Mr. Dab returned 14,465,259 shares of Common Stock in InnovaQor for cancellation.
The goal of the Company is to develop and deliver a technology-based communication platform to a broad range of healthcare professionals and businesses using a subscription revenue model with added value bolt on services.
InnovaQor has six wholly-owned subsidiaries that provide medical support services primarily to clinical laboratories, corporate operations, rural hospitals, physician practices and behavioral health/substance abuse centers.
Health Technology Solutions, Inc. (“HTS”): HTS provides information technology and software solutions to our subsidiaries and outside medical service providers. HTS provides vCIO, IT managed services and data analytics dashboards to our subsidiaries and outside medical service providers. HTS operates from the corporate offices in West Palm Beach, Florida.
Medical Mime, Inc. (“Mime”): Mime was formed on May 9, 2014. It specializes in electronic health records (EHR) software and subscription services for the behavioral health and rehabilitation market segments. It currently serves nine behavioral health/substance abuse facilities.
ClinLab, Inc. (“ClinLab”): ClinLab develops and markets laboratory information management systems to mid-size clinical laboratories. It currently services 12 clinical laboratories across the country.
AMSG owns CollabRx, Inc. (“CollabRx”) and Genomas, Inc. (“Genomas”), each of which is an inactive operation.
Genomas operated a diagnostics lab until December 31, 2019 and was focused solely on the pharmacogenomics technology and platform, MedTuning, to interpret diagnostics outcomes and translate these outcomes into easily usable information to indicate the effectiveness of medications for a patient. This solution would require minimum effort to be back in operation. CollabRx owns a technology platform and database for interpreting diagnostics outcomes from cancer patients that could match the result to known treatments and or clinical trials. This solution has been dormant for a number of years and to be viable in the marketplace will require updates to the technology and the database.
| F-8 |
Each of the subsidiaries is wholly owned by the Company and complements each other, allowing for cross selling of products and services. The Company believes the current solutions will become an added value option to a technology-based communication platform to a broad range of healthcare professionals and businesses using a subscription revenue model with added value bolt on services, the Company plans to develop.
Existing products offered by the Company’s subsidiaries are as follows:
“M2Select” is a custom built, cloud based, electronic health record which meets the needs of substance abuse treatment and behavioral health providers. M2Select’s specialized clinical workflow provides intuitive prompts for symptoms and enables you to quickly select problems and create master treatment plans with goals, objectives, and interventions. M2Select provides best-in-class patient lifecycle management for Behavioral Health/Substance Abuse (BH/SA) treatment centers. From pre-admission to billing and aftercare, M2Select is an electronic health record and patient management software that seamlessly integrates into the natural workflow of day-to-day operations.
“M2Pro” is a custom built, cloud based, electronic health record for ambulatory physician practices that meets meaningful use stage 2 and no further. Its unique dictation services further automate the workflow process for physicians allowing them to focus on their continuum of patient care.
“ClinLab” is a turnkey client/server lab information system for mid-range laboratories. ClinLab supports interfaces to all major reference labs and the ClinLab team can provide an interface to any system with that capability. ClinLab also features an optional EHR package which enables interfacing with the most popular EHR systems allowing lab test results to integrate seamlessly into a provider’s EHR for an improved patient record and to fulfill the federal government requirements.
“Qira” is our healthcare business analytics service powered by PowerBI. It is a culmination of healthcare financial and revenue cycle management plus clinical operations oversight needs. It aggregates data from multiple healthcare systems to produce a single source business intelligence tool with executive level daily briefing to deep dive operational management of claims and operational efficiencies. There are many other analytical services available that customize solutions but none that have a proven template for success. Our competitive advantage comes from having created these tools to identify the deficiencies in the real world for the former parent Rennova from its former national laboratory operations to its more recent rural hospitals.
“vCIO Services”. Based on the skills and experience inherent within InnovaQor and resulting from work undertaken on behalf of the former parent, Rennova, InnovaQor offers a range of CIO services centered on our ability to link IT systems to business objectives combined with our knowledge of technology trends likely to impact our sector. The CIO services would include (but not be limited to):
| ● | Program and Project Management | |
| ● | Vendor Management | |
| ● | Business Continuity and Disaster Recovery | |
| ● | Security Services | |
| ● | Network Infrastructure Management | |
| ● | Helpdesk Provision |
| F-9 |
“MedTuning” utilizes proprietary biomarkers, treatment algorithms, and a web-based interactive physician portal delivery system to provide clinical decision support for physicians and personalized drug treatment for patients. Products are DNA-guided to improve the therapeutic benefit of widely used prescription drugs while also reducing the risk of significant side effects for patients.
Medical Informatics: Our technology platform, proprietary algorithms and physician interface portal can be extended to a wide range of drug categories.
Research and Development: Technology platform applicable to numerous disease states; current pipeline in mental health, pain management, cardiovascular and diabetes.
“Advantage” is a proprietary HIPAA compliant software developed to eliminate the need for paper requisitions by providing an easy to use and efficient web-based system that lets customers securely place lab orders, track samples and view test reports in real time from any web-enabled laptop, notepad or smart phone.
In the coming year we plan to develop, acquire or license and offer a telehealth solution through corporate partnerships in the emerging health technology sector.
Note 2 - Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The acquisition of an operating company by a non-operating public shell corporation typically results in the owners and management of the operating company having actual or effective voting and operating control of the combined company. The Securities and Exchange Commission staff considers a public shell reverse acquisition to be a capital transaction in substance, rather than a business combination. That is, the transaction is a reverse recapitalization, equivalent to the issuance of stock by the operating company for the net monetary assets of the shell corporation accompanied by a recapitalization. The accounting is similar to that resulting from a reverse acquisition, except that no goodwill or other intangible assets are recorded.
The consolidated financial statements include the accounts of only the HTS Group (the accounting acquirer) prior to June 25, 2021 and InnovaQor and the Group since the date of acquisition on June 25, 2021, with the transaction being accounted for as a recapitalization of the Group on June 25, 2021. The consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and require management to make certain judgments, estimates, and assumptions. These may affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements. They also may affect the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates upon subsequent resolution of identified matters.
| F-10 |
Comprehensive Loss
During the years ended December 31, 2021 and 2020, comprehensive loss was equal to the net loss amounts presented in the accompanying condensed consolidated statements of operations.
Going Concern
Under Accounting Standards Update (“ASU”) 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40) Accounting Standards Codification (“ASC 205-40”), the Company has the responsibility to evaluate whether conditions and/or events raise substantial doubt about its ability to meet its future financial obligations as they become due within one year after the date that the financial statements are issued. As required by ASC 205-40, this evaluation shall initially not take into consideration the potential mitigating effects of plans that have not been fully implemented as of the date the financial statements are issued. Management has assessed the Company’s ability to continue as a going concern in accordance with the requirement of ASC 205-40.
The accompanying consolidated financial statements have been prepared in accordance with U.S. GAAP and the rules and regulations of the SEC. The consolidated financial statements have been prepared using U.S. GAAP applicable to a going concern that contemplates the realization of assets and liquidation of liabilities in the normal course of business. The Company has accumulated significant losses and has negative cash flows from operations and, at December 31, 2021, had a working capital deficit and accumulated deficit of $2.9 million and $18.0 million, respectively. In addition, the Company’s cash position is critically deficient and critical payments are not being made in the ordinary course of business, all of which raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans with respect to alleviating the adverse financial conditions that caused management to express substantial doubt about the Company’s ability to continue as a going concern are as follows:
The Company will incur substantial costs in connection with the acquisition, which may include accounting, tax, legal and other professional services costs, recruiting and relocation costs associated with hiring key senior management personnel who are new to the Company, tax costs and costs to separate information systems, among other costs. The cost of performing such functions is anticipated to be higher than the amounts reflected in the Company’s historical financial statements, which would cause its future losses to increase. Accordingly, the Company will continue to focus on reducing its operating costs and increasing revenues.
There can be no assurance that the Company will be able to achieve its business plan, raise any additional capital or secure the additional financing necessary to implement its current operating plan. The ability of the Company to continue as a going concern is dependent upon its ability to increase its revenues and eventually achieve profitable operations. The accompanying consolidated financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant areas of estimation include estimating the fair value of intangible assets acquired, the impairment of assets, accrued and contingent liabilities, and future income tax obligations (benefits), among other items. Actual results could differ from those estimates and would impact future results of operations and cash flows.
| F-11 |
Cash and Cash Equivalents
The Company considers all highly liquid temporary cash investments with an original maturity of three months or less to be cash equivalents.
Allowance for Doubtful Accounts Policy
Accounts receivable are reported at realizable value, net of allowances for doubtful accounts, which are estimated and recorded in the period that the Company deems the receivable to be uncollectable. The Company has a standardized approach to estimate and review the collectability of its receivables based on a number of factors, including the period they have been outstanding. Historical collection is an integral part of the estimation process related to the allowance for doubtful accounts. In addition, the Company regularly assesses the state of its billing operations in order to identify issues that may impact the collectability of these receivables or reserve estimates. Receivables deemed to be uncollectible are charged against the allowance for doubtful accounts at the time such receivables are written-off. Recoveries of receivables previously written-off are recorded as credits to the allowance for doubtful accounts. Revisions to the allowances for doubtful accounts estimates are recorded as an adjustment to the provision for bad debts.
Revenue Recognition
We recognize revenue in accordance with Accounting Standards Update (“ASU”) 2014-09, “Revenue from Contracts with Customers (Topic 606),” including subsequently issued updates. This series of comprehensive guidance has replaced all existing revenue recognition guidance. There is a five-step approach outlined in the standard. In determining revenue, we first identify the contract according to the scope of ASU Topic 606 with the following criteria:
| ● | Identify the contract(s) with a customer. | |
| ● | Identify the performance obligations in the contract. | |
| ● | Determine the transaction price. | |
| ● | Allocate the transaction price to the performance obligations in the contract. | |
| ● | Recognize revenue when or as you satisfy a performance obligation. |
Revenue is recognized when control of the promised services is transferred to the Company’s customers in an amount that reflects the consideration the Company is expected to be entitled to in exchange for those services. As the Company completes its performance obligations which are identified in Note 11 below, it has an unconditional right to consideration as outlined in the Company’s contracts. Generally, the Company’s accounts receivable are expected to be collected in 30 days in accordance with the underlying payment terms. For many of the Company’s services, the Company typically has one performance obligation; however, it also provides the customer with an option to acquire additional services. The Company typically provides a menu of offerings from which the customer may choose to purchase. The price of each service is generally based upon an agreed hourly rate.
Impairment or Disposal of Long-Lived Assets
The Company accounts for the impairment or disposal of long-lived assets according to the Financial Accounting Standards Board’s (“FASB”) ASC 360, “Property, Plant and Equipment.” Long-lived assets are reviewed when facts and circumstances indicate that the carrying value of the asset may not be recoverable. When necessary, impaired assets are written down to estimated fair value based on the best information available. Estimated fair value is generally based on either appraised value or measured by discounting estimated future cash flows. Considerable management judgment is necessary to estimate discounted future cash flows. Accordingly, actual results could vary significantly from such estimates. As of December 31, 2021 and 2020, the majority of the Company’s fixed assets were fully depreciated and, therefore, the carrying value of fixed assets represented fair value. Fixed assets are depreciated over lives ranging from three to seven years.
Fair Value of Financial Instruments
In accordance with ASC 820, “Fair Value Measurements and Disclosures,” the Company applies fair value accounting for all financial assets and liabilities and non-financial assets and liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis. Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities that are required to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and the market-based risk measurements or assumptions that market participants would use in pricing the asset or liability, such as risks inherent in valuation techniques, transfer restrictions and credit risk. Fair value is estimated by applying the following hierarchy, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement:
| ● | Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. | |
| ● | Level 2 applies to assets or liabilities for which there are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly, such as quoted prices for similar assets or liabilities in active markets; or quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets). | |
| ● | Level 3 applies to assets or liabilities for which fair value is derived from valuation techniques in which one or more significant inputs are unobservable, including the Company’s own assumptions. |
| F-12 |
The estimated fair value of financial instruments is determined by the Company using available market information and valuation methodologies considered to be appropriate. At December 31, 2021 and 2020, the carrying value of the Company’s accounts receivable, accounts payable, accrued expenses and notes payable, approximate their fair values due to their short-term nature. For the years ended December 31, 2021 and 2020, there were no realized and unrealized gains on instruments valued using fair value evaluation methods.
Income Taxes
The entities within the Group were included in the consolidated income tax returns of its Parent for the years ended December 31, 2020 and prior. A determination has been made by Parent’s management not to allocate any of the deferred tax assets or liabilities to the Group as of December 31, 2020 and prior. Accordingly, the Group had not provided for income taxes in the combined financial statements. The Company since June 25, 2021 uses the liability method of accounting for income taxes. Under the liability method, future tax liabilities and assets are recognized for the estimated future tax consequences attributable to differences between the amounts reported in the financial statement carrying amounts of assets and liabilities and their respective tax bases. Future tax assets and liabilities are measured using enacted or substantially enacted income tax rates expected to apply when the asset is realized or the liability settled. The effect of a change in income tax rates on future income tax liabilities and assets is recognized in income in the period that the change occurs. Future income tax assets are recognized to the extent that they are considered more likely than not to be realized. When projected future taxable income is insufficient to provide for the realization of deferred tax assets, the Company will recognize a valuation allowance.
In accordance with U.S. GAAP, the Company has determined whether a tax position of the Company is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The tax benefit to be recognized is measured as the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement. Derecognition of a tax benefit previously recognized could result in the Company recording a tax liability that would reduce net assets. The Company has determined that it has not incurred any liability for tax benefits as of December 31, 2021 and 2020. State income taxes will also be due on any income generated in the future.
| F-13 |
Convertible Preferred Stock
The Company classifies its Series B-1 and Series C-1 Convertible Preferred Stock as liabilities in accordance with ASC 480 Distinguishing Liabilities from Equity since the preferred stock is convertible, at the option of the holder, into a variable number of shares based solely on a fixed dollar amount (stated value) known at issuance of the preferred stock.
Basic and Diluted Net Income (Loss) Per Share
The Company computes net income (loss) per share in accordance with ASC Topic 260, “Earnings per Share” which requires presentation of both basic and diluted earnings per share (EPS) on the face of the income statement. Basic EPS is computed by dividing net income (loss) available to common shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period. Diluted EPS gives effect to all dilutive potential common shares outstanding during the period including stock options, using the treasury stock method, and convertible preferred stock, using the if-converted method. As of December 31, 2021 and 2020, there were approximately 1,773,000,000 and 0 common stock equivalents, respectively, which where antidilutive due to the Company’s losses. These common stock equivalents are in connection with the convertible preferred stock.
Note 3 – Acquisition
The Company acquired all the common stock of the HTS Group from Rennova on June 25, 2021 in exchange for Preferred Series A-1, B-1 and C-1 stock with a fair market value of $9,195,692. This acquisition has been accounted for as a reverse acquisition with the HTS Group being the accounting acquiror with the excess fair value of the purchase price over net asset fair value acquired treated as a reduction of additional paid in capital on the date of acquisition.
A summary of that purchase price is a s follows:
Schedule of Purchase Price
| Fair Value of Preferred Series A-1 Stock | $ | 100 | ||
| Fair Value of Preferred Series B-1 Stock | $ | 9,086,396 | ||
| Fair Value of Preferred Series C-1 Stock | $ | 122,000 | ||
| Other | $ | (12,804 | ) | |
| Total | $ | 9,195,692 |
On the date of acquisition, InnovaQor had no assets and total liabilities of $500,035, of which $500,000 was converted to equity in connection with the acquisition.
The following is an unaudited summary Statement of Operations as if the acquisition of the Group had taken place on July 1, 2020:
Summary of Statement of Operations Acquisition
| Years Ended | ||||||||
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Net revenues | $ | 468,883 | $ | 528,624 | ||||
| Operating expenses: | ||||||||
| Direct costs of revenue | 205,649 | 5,536 | ||||||
| General and administrative expenses | 1,755,455 | 1,061,155 | ||||||
| Depreciation | 1,185 | 2,168 | ||||||
| Total operating expenses | 1,962,289 | 1,068,859 | ||||||
| Loss from operations | (1,493,406 | ) | (540,235 | ) | ||||
| Other (expense) income | 139,795 | (84,129 | ) | |||||
| Loss before income taxes | (1,353,611 | ) | (624,364 | ) | ||||
| Provision for income taxes | - | - | ||||||
| Net loss | $ | (1,353,611 | ) | $ | (624,364 | ) | ||
| F-14 |
Note 4 – Accounts Receivable
Accounts receivable at December 31, 2021 and 2020 consisted of the following:
Schedule of Accounts Receivable
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Accounts receivable (including related party of $21,852 and $135,687 at December 31, 2021 and 2020, respectively) | $ | 32,800 | $ | 152,759 | ||||
| Less: | ||||||||
| Allowance for discounts | — | (1,396 | ) | |||||
| Accounts receivable, net | $ | 32,800 | $ | 151,363 | ||||
For the years ended December 31, 2021 and 2020, bad debt expense was $6,554 and $16,165, respectively.
Note 5 – Property and Equipment
Property and equipment at December 31, 2021 and 2020 consisted of the following:
Summary of Property and Equipment
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Software | $ | 1,435,875 | $ | 1,435,875 | ||||
| Furniture | 8,227 | 8,227 | ||||||
| Office equipment | 30,931 | 24,883 | ||||||
| Computer equipment | 324,131 | 330,179 | ||||||
| 1,799,164 | 1,799,164 | |||||||
| Less accumulated depreciation | (1,799,164 | ) | (1,798,479 | ) | ||||
| Property and equipment, net | $ | — | $ | 685 | ||||
Depreciation expense on property and equipment was $1,185 and $2,168 for the years ended December 30, 2021 and 2020, respectively. Management periodically reviews the valuation of long-lived assets, including property and equipment, for potential impairment.
| F-15 |
Note 6 – Accrued Expenses
Accrued expenses at December 31, 2021 and 2020 consisted of the following:
Schedule of Accrued Expenses
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Accrued payroll and related liabilities | $ | 1,263,539 | $ | 1,173,889 | ||||
| Accrued legal | 37,997 | 37,997 | ||||||
| Accrued interest | 17,494 | 681 | ||||||
| Deferred revenue and customer deposits | 24,471 | 19,530 | ||||||
| Other accrued expenses | 103,221 | 76,186 | ||||||
| Accrued expenses | $ | 1,446,722 | $ | 1,308,283 | ||||
Accrued payroll and related liabilities at December 31, 2021 and 2020 included approximately $1.1 million and $1.1 million, respectively, of accrued past due payroll taxes, related penalties and interest.
Note 7 – Notes Payable
The carrying amount of notes payable as of December 31, 2021 and 2020 was as follows:
Schedule of Notes Payable
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Note payable with the Department of Economic and Community Development in the original amount of $147,372 due in monthly payments of principal and interest totaling $2,132 beginning January 1, 2017 with a final payment due on October 1, 2022. Non-interest bearing. Payments were not made in 2021 or 2020. | $ | 134,153 | $ | 134,118 | ||||
| Paycheck Protection Program Loans (PPP Loans). The PPP Loans and accrued interest are forgivable as long as the Company uses the loan proceeds for eligible purposes, including payroll, benefits, rent and utilities, and maintains its payroll levels. The amount of loan forgiveness will be reduced if the Company terminates employees or reduces salaries. No collateral or guarantees were provided in connection with the PPP Loans. The unforgiven portion of the PPP Loans are payable over two years at an interest rate of 1.0% per annum, with a deferral of payments for the first sixteen months. Beginning sixteen months from the dates of issuance, the Company is required (if not forgiven) to make monthly payments of principal and interest to the lenders. The Company intends to use all of the proceeds for purposes consistent with the PPP. While the Company currently believes that its use of the loan proceeds will meet the conditions for forgiveness of the loans, it cannot assure you that it will not take actions that could cause the Company to be ineligible for forgiveness of the loans, in whole or in part. | 60,401 | 103,900 | ||||||
| 194,554 | 238,018 | |||||||
| Less current portion | 134,153 | 134,118 | ||||||
| Notes payable, long term, net of current portion | $ | 60,401 | $ | 103,900 | ||||
In July 2021, Evolve Bank and Trust Company and the Small Business Administration forgave $103,900 of Paycheck Protection Program Loans (PPP Loans) and related interest.
| F-16 |
Note 8 – Loans from Parent and Other Related Party Transactions
To fund the Company’s operations for the years ended December 31, 2021 and 2020, the former Parent advanced funds and paid expenses of InnovaQor and the Group in the amount of $374,473 and $417,349, respectively, which is shown in the accompanying Consolidated Balance Sheets as Due to Former Parent as of December 31, 2021 and in Parent Net Investment Deficit in Advanced Molecular and Health Technology Solutions Group as of December 31, 2020.
Related Party Revenue
Included in net revenues for the years ended December 31, 2021 and 2020 is $237,551 and $185,892, respectively, of revenue with Rennova (the former parent).
The Group has incurred certain costs that have been allocated from Rennova. Included in the Consolidated Statements of Operations are the following allocated costs:
Schedule of Allocated Costs
Year Ended December 31, | Year Ended December 31, | |||||||
| 2021 | 2020 | |||||||
| Health insurance | $ | 16,169 | $ | 2,825 | ||||
| Rent and utilities | 83,429 | 27,343 | ||||||
| Total allocated costs | $ | 99,598 | $ | 30,168 | ||||
Note 9 – Preferred Stock and Stockholders’ Deficit
Common Stock
The Company has authorized 325,000,000 shares of $0.0001 par value Common Stock of which 234,953,286 were issued and outstanding as of December 31, 2021. These shares have one vote per share.
Preferred Stock Series A-1
The Company has authorized 1,000 shares of $0.0001 par value (stated value $10) Series A-1 Supermajority Voting Preferred Stock of which 1,000 were issued and outstanding as of December 31, 2021. So long as one share of Series A-1 Preferred Stock is outstanding, the outstanding shares of the Series A-1 Preferred Stock shall have the number of votes, in the aggregate, equal to 51% of all votes entitled to be voted at any stockholder meeting. These shares have no rights to receive dividends and liquidation rights are equal to the stated value per share.
Preferred Stock Series B-1
The Company has authorized 25,000 shares of $0.0001 par value (stated value $1,000) Series B-1 Convertible Redeemable Preferred Stock of which 14,950 were issued and outstanding as of December 31, 2021. These shares have no voting rights, dividends on these shares shall accrue at the rate of 5% of the stated value per share and liquidation rights are equal to the stated value per share. These shares are convertible into the Company’s Common Stock based on the stated value at a conversion price equal to 90% of the average closing price of the Common Stock on the 10 Trading Days immediately prior to the Conversion Date but in any event no less than the par value of the Common Stock. The Series B-1 Preferred Stock was not convertible prior to the first anniversary of its issuance except with the consent of the holders of a majority of the then outstanding shares, if any, of the Series A-1 Preferred Stock. No conversion can be made to the extent the holder’s beneficial interest (as defined pursuant to the terms of the Series B-1 Preferred Stock) in the common stock of InnovaQor would exceed 4.99%. These shares are redeemable at the option of the Company at their stated value plus declared and unpaid dividends. Because these shares are convertible, at the option of the holder, into a variable number of common shares based solely on a fixed dollar amount (stated value) known at issuance of the shares, they have been recorded as a long-term liability at the date of issuance in accordance with ASC 480, Distinguishing Liabilities from Equity.
| F-17 |
Preferred Stock Series C-1
The Company has authorized 2,000 shares of $0.0001 par value (stated value $1,000) Series C-1 Convertible Redeemable Preferred Stock of which 200 are issued and outstanding as of December 31, 2021. These shares have no voting rights, dividends on these shares shall accrue at the rate of 10% of the stated value per share and liquidation rights are equal to the stated value per share. These shares are convertible into the Company’s Common Stock based on the stated value at a conversion price equal to 90% of the average closing price of the Common Stock on the 10 Trading Days immediately prior to the Conversion Date but in any event no less than the par value of the Common Stock. The Series C-1 Preferred Stock was not convertible prior to the first anniversary of its original issuance except with the consent of the holders of a majority of the then outstanding shares, if any, of the Series A-1 Preferred Stock. No conversion can be made to the extent the holder’s beneficial interest (as defined pursuant to the terms of the Series C-1 Preferred Stock) in the common stock of InnovaQor would exceed 4.99%. These shares are redeemable at the option of the Company at their stated value plus declared and unpaid dividends. Because these shares are convertible, at the option of the holder, into a variable number of common shares based solely on a fixed dollar amount (stated value) known at issuance of the shares, they have been recorded as a long-term liability at the date of issuance in accordance with ASC 480, Distinguishing Liabilities from Equity.
Note 10 – Revenue
The Company had net revenue for the years ended December 31, 2021 and 2020 as follows:
Schedule of Net Revenue
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Dashboards | $ | 69,258 | $ | — | ||||
| IT Managed Services | 102,960 | — | ||||||
| Software and Interfaces | 58,599 | 15,015 | ||||||
| Support and Maintenance | 95,355 | 299,082 | ||||||
| vCIO Services | 65,313 | — | ||||||
| Software Licenses Fees | 74,618 | 204,124 | ||||||
| Other | 2,780 | 10,403 | ||||||
| Total Net Revenue | $ | 468,883 | $ | 528,624 | ||||
Generally, work is billed monthly by the hour at agreed upon hourly rates for all of the above revenue streams.
For all of the Company’s services, the Company typically has one performance obligation; however, it also provides the customer with an option to acquire additional services. The Company typically provides a menu of offerings from which the customer may choose to purchase. The price of each service is separate and distinct and provides a separate and distinct value to the customer. Pricing is generally consistent for each service irrespective of the other services or quantities requested by the customer.
When the Company receives consideration from a customer prior to transferring services to the customer under the terms of the contract, it records deferred revenues on the Company’s consolidated balance sheet, which represents a contract liability.
The Company has an internal sales force compensation program where remuneration is based solely on the revenues recognized in the period and does not represent an incremental cost to the Company which provides a future benefit expected to be longer than one year and would meet the criteria to be capitalized and presented as a contract asset on the Company’s consolidated balance sheet.
Note 11 – Other Related Party Transactions
Mr. Dab, the former CEO of InnovaQor, paid some of the disbursements on behalf of the Company for the years ended December 31, 2021 and 2020. The disbursements amounted to $24,993 and $7,670, respectively, and are shown in the accompanying Consolidated Statements of Operations.
| F-18 |
In the years ended December 31, 2021 and 2020, the former Parent advanced funds and paid expenses of InnovaQor and the Group in the amount of $374,473 and $417,349, respectively, which is shown in the accompanying Consolidated Balance Sheets as Due to Former Parent as of December 31, 2021 and in Parent Net Investment Deficit in Advanced Molecular and Health Technology Solutions Group as of December 31, 2020.
The above amounts are not indicative of what third parties would have agreed to.
Note 12 – Commitments and Contingencies
Consulting Agreement – the Company entered into a consulting agreement effective June 1, 2021, with Dial M Productions LLC, a company owned by Mr. Dab, the Company’s former CEO, for a period of one year to provide assistance in developing the Company’s business including communications with existing shareholders and the general public. This company shall be paid $60,000 upon receipt of funding from an outside source or within 90 days of signing the agreement. This has not yet been paid. This agreement has been renewed for another year. Additionally, this company shall be paid $3,500 per month until the agreement expires.
Concentration of Credit Risk - Credit risk with respect to accounts receivable is generally low due to the nature of the customers comprising the customer base and the significant related party component. The Company does not require collateral or other security to support customer receivables. However, the Company continually monitors and evaluates its client acceptance and collection procedures to minimize potential credit risks associated with its accounts receivable and establishes an allowance for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is not material to the consolidated financial statements.
The Company maintains its cash balances in high-credit-quality financial institutions. The Company’s cash balances may, at times, exceed the deposit insurance limits provided by the Federal Deposit Insurance Corp.
Guarantees
Certain subsidiaries of the Company have guaranteed debt obligations of their former Parent. As part of the transaction with the Company, the former Parent received a release of guarantees from certain institutional lenders and has been working to settle other debt obligations where certain subsidiaries of the Company remain a guarantor. The Company believes that any risk associated with previous guarantees is now minimal and immaterial.
Legal Matters
From time to time, the Company may be involved in a variety of claims, lawsuits, investigations and proceedings related to contractual disputes, employment matters, regulatory and compliance matters, intellectual property rights and other litigation arising in the ordinary course of business. The Company operates in a highly regulated industry which may inherently lend itself to legal matters. Management is aware that litigation has associated costs and that results of adverse litigation verdicts could have a material effect on the Company’s condensed consolidated financial position or results of operations. Management, in consultation with legal counsel, has addressed known assertions and predicted unasserted claims below.
P2P Staffing Corp. received a judgment against HTS during 2018 in the amount of $58,784 plus accrued interest and court costs for amounts owed. As of December 31, 2021 and 2020, $10,464 was outstanding and owed for this judgment and included in accounts payable in the accompanying Consolidated Balance Sheets.
Two former employees of CollabRx, Inc., one of the acquired subsidiaries, filed suits in a California state court against the former Parent, Rennova, and CollabRx, Inc., in connection with amounts claimed to be owed under their respective employment agreements with CollabRx, Inc. One former employee received a judgment for approximately $253,000, which Rennova has paid in full. The other former employee received a judgment for approximately $173,000.
| F-19 |
ClinLab, Medical Mime and HTS, as well as the former Parent, Rennova and many of its subsidiaries, were defendants in a case filed in Broward County Circuit Court by TCA Global Credit Master Fund, L.P. The plaintiff alleged a breach by Medytox Solutions, Inc. of its obligations under a debenture and claimed damages of approximately $2,030,000 plus interest, costs and fees. The other entities were sued as alleged guarantors of the debenture. In May 2020, the SEC appointed a Receiver to close down the TCA Global Credit Master Fund, L.P. In September 2021, the parties entered into a settlement agreement with the Receiver to pay $500,000 as full and final settlement of all claims in this matter, which amount has now been paid as of April 2022.
CTI Consulting LLC filed suit against HTS in September 2021, claiming approximately $45,000 as owed for services provided. The Company has agreed to pay $5,000 per month until the obligation is satisfied.
On June 14, 2018, Techlogix, Inc. obtained a judgment in the Massachusetts Superior Court for Middlesex County in the amount of $71,223 against HTS and Rennova. This amount was paid by Rennova and a Satisfaction of Judgement was filed by Techlogix, Inc. on December 8, 2020.
Note 13 – Supplemental Disclosure of Cash Flow Information
Schedule of Supplemental Disclosure of Cash Flow Information
| Years ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Cash paid for interest | $ | 1,066 | $ | 174,551 | ||||
| Cash paid for income taxes | $ | — | $ | — | ||||
| Series C-1 Preferred Stock issued in connection with acquisition | $ | 122,000 | — | |||||
| Series B-1 Preferred Stock issued in connection with acquisition | $ | 9,086,396 | — | |||||
Note 14 – Recent Accounting Pronouncements
All recent accounting standards issued by the FASB, including its Emerging Issues Task Force, the American Institute of Certified Public Accountants, and the SEC did not or are not believed by management to have a material impact on the Company’s consolidated financial statements.
| F-20 |
CONDENSED CONSOLIDATED BALANCE SHEETS
| June 30, | December 31, | |||||||
| 2022 | 2021 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 6,598 | $ | 46 | ||||
| Accounts receivable, net (including related party receivable of $12,327 and $21,852 at June 30, 2022 and December 31, 2021, respectively) | 52,837 | 32,800 | ||||||
| Prepaid expenses and other current assets | 7,718 | — | ||||||
| Total current assets | 67,153 | 32,846 | ||||||
| Property and equipment, net | — | — | ||||||
| Total assets | $ | 67,153 | $ | 32,846 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 1,053,375 | $ | 937,698 | ||||
| Accrued expenses | 1,437,870 | 1,446,722 | ||||||
| Due to Former Parent | 803,416 | 374,473 | ||||||
| Current portion of notes payable | 223,952 | 134,153 | ||||||
| Total current liabilities | 3,518,613 | 2,893,046 | ||||||
| Notes payable – Long term | 60,401 | 60,401 | ||||||
| — | ||||||||
| Preferred Series B-1 Stock, Par Value $0.0001, 25,000 shares authorized, 14,950 shares issued and outstanding at June 30, 2022 and December 31, 2021 | 9,086,396 | 9,086,396 | ||||||
| Preferred Series C-1 Stock, Par Value $0.0001, 2,000 shares authorized, 225 and 200 shares issued and outstanding at June 30, 2022 and December 31, 2021, respectively | 137,250 | 122,000 | ||||||
| Total temporary capital | ||||||||
| Total liabilities | 12,802,660 | 12,161,843 | ||||||
| Commitments and contingencies | - | - | ||||||
| Stockholders’ Deficit | ||||||||
| Preferred Series A-1 Stock, Par Value $0.0001, 1,000 shares authorized, 1,000 shares issued and outstanding at June 30, 2022 and December 31, 2021 | — | — | ||||||
| Common Stock, Par Value $0.0001, 325,000,000 shares authorized, 234,953,286 shares issued and outstanding at June 30, 2022 and December 31, 2021 | 23,495 | 23,495 | ||||||
| Additional Paid-In Capital | 5,857,658 | 5,857,658 | ||||||
| Accumulated Deficit | (18,616,660 | ) | (18,010,150 | ) | ||||
| Total Stockholders’ Deficit | (12,735,507 | ) | (12,128,997 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 67,153 | $ | 32,846 | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| F-21 |
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
| Three Months Ended | ||||||||
| June 30, 2022 | June 30, 2021 | |||||||
| Net revenues (including net revenue from related party of $47,788 and $62,316 for the three months ended June 30, 2022 and 2021, respectively) | $ | 94,511 | $ | 104,676 | ||||
| Operating expenses: | ||||||||
| Direct costs of revenue | 80,768 | 1,996 | ||||||
| General and administrative expenses | 337,695 | 272,644 | ||||||
| Total operating expenses | 418,463 | 274,640 | ||||||
| Loss from operations | $ | (323,952 | ) | $ | (169,964 | ) | ||
| Other (expense) income: | ||||||||
| Other (expense) income, net | — | — | ||||||
| Interest expense | (17,835 | ) | (328 | ) | ||||
| Total other (expense) income | (17,835 | ) | (328 | ) | ||||
| Loss before income taxes | (341,787 | ) | (170,292 | ) | ||||
| Provision for income taxes | — | — | ||||||
| Net loss | $ | (341,787 | ) | $ | (170,292 | ) | ||
| Basic and Diluted (loss) per share | $ | (0.00 | ) | (0.00 | ) | |||
| Basic and Diluted weighted average shares of common stock outstanding | 234,953,286 | 234,953,286 | ||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| F-22 |
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
| Six Months Ended | ||||||||
| June 30, 2022 | June 30, 2021 | |||||||
| Net revenues (including net revenue from related party of $102,267 and $124,632 for the six months ended June 30, 2022 and 2021, respectively) | $ | 191,327 | $ | 216,941 | ||||
| Operating expenses: | ||||||||
| Direct costs of revenue | 255,856 | 2,386 | ||||||
| General and administrative expenses | 520,974 | 551,203 | ||||||
| Total operating expenses | 776,830 | 553,589 | ||||||
| Loss from operations | $ | (585,503 | ) | $ | (336,648 | ) | ||
| Other (expense) income: | ||||||||
| Other (expense) income, net | — | — | ||||||
| Interest expense | (21,007 | ) | (9,577 | ) | ||||
| Total other (expense) income | (21,007 | ) | (9,577 | ) | ||||
| Loss before income taxes | (606,510 | ) | (346,225 | ) | ||||
| Provision for income taxes | — | — | ||||||
| Net loss | $ | (606,510 | ) | $ | (346,225 | ) | ||
| Basic and Diluted (loss) per share | $ | (0.00 | ) | $ | (0.00 | ) | ||
| Basic and Diluted weighted average shares of common stock outstanding | 234,953,286 | 234,953,286 | ||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| F-23 |
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
(Unaudited)
| Preferred Series A-1 Shares | Common Stock | Additional Paid-in | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Total | ||||||||||||||||||||||
| December 31, 2020 | - | $ | - | - | $ | - | $ | 15,076,845 | $ | (17,164,307 | ) | $ | (2,087,462 | ) | ||||||||||||||
| Issuance of Stock in Transaction | 1,000 | - | 234,953,286 | $ | 23,495 | (23,495 | ) | - | - | |||||||||||||||||||
| Issuance of Preferred Stock as part of reverse merger | - | - | - | - | (9,195,692 | ) | - | (9,195,692 | ) | |||||||||||||||||||
| Net loss | - | - | - | - | - | (346,225 | ) | (346,225 | ) | |||||||||||||||||||
| June 30, 2021 | 1,000 | $ | - | 234,953,286 | $ | 23,495 | $ | 5,857,658 | $ | (17,510,532 | ) | $ | (11,629,379 | ) | ||||||||||||||
| December 31, 2021 | 1,000 | $ | - | 234,953,286 | $ | 23,495 | $ | 5,857,658 | $ | (18,010,150 | ) | $ | (12,128,997 | ) | ||||||||||||||
| Net loss | - | - | - | - | - | (606,510 | ) | (606,510 | ) | |||||||||||||||||||
| June 30, 2022 | 1,000 | $ | - | 234,953,286 | $ | 23,495 | $ | 5,857,658 | $ | (18,616,660 | ) | $ | (12,735,507 | ) | ||||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| F-24 |
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
| Six Months Ended | ||||||||
| June 30, 2022 | June 30, 2021 | |||||||
| Cash flows (used in) operating activities: | ||||||||
| Net loss | $ | (606,510 | ) | $ | (346,225 | ) | ||
| Adjustments to reconcile net loss to net cash (used in) operations: | ||||||||
| Amortization of Debt Discount | 14,949 | — | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (20,037 | ) | 129,446 | |||||
| Prepaid expenses | (7,718 | ) | 859 | |||||
| Accounts payable and accrued expenses | 106,825 | 111,771 | ||||||
| Net cash (used in) operating activities | (512,491 | ) | (104,149 | ) | ||||
| Cash flows (used in) investing activities: | ||||||||
| Purchase of fixed assets | — | — | ||||||
| Net cash (used in) investing activities | — | — | ||||||
| Cash flows provided by financing activities: | ||||||||
| Loans from Former Parent | 428,943 | 12,500 | ||||||
| Loan from CEO | 90,100 | — | ||||||
| Proceeds from Paycheck Protection Program Loans | — | 60,401 | ||||||
| Net cash provided by financing activities | 519,043 | 72,901 | ||||||
| Net (Decrease) Increase in cash | 6,552 | (31,248 | ) | |||||
| Cash at beginning of period | 46 | 31,294 | ||||||
| Cash at end of period | $ | 6,598 | $ | 46 | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| F-25 |
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Note 1 – Description of Business
InnovaQor, Inc. (which changed its name from VisualMED Clinical Solutions Corporation in September 2021) (“InnovaQor” or the “Company”) was incorporated in the State of Nevada on September 7, 1999. Its business plan involved the distribution of medical software. It was primarily involved in activities related to the distribution of medical software through associated companies to which it has granted operating and distribution licenses.
During 2017, Rennova Health, Inc. (“Rennova” or the “Parent”), the Parent of Advanced Molecular Services Group, Inc. (“AMSG”) and Health Technology Solutions, Inc. (“HTS”) (collectively, the “Advanced Molecular and Health Technology Solutions Group,” or the “Group”), announced its intent to separate the Group into one or more separate public entities with AMSG holding and operating Rennova’s pharmacogenomics business and HTS holding and operating Rennova’s supportive software solutions business. Pharmacogenomics is the genetic process to understand how an individual’s genetic attributes affect the likely response to therapeutic drugs. HTS’s supportive software solutions business includes electronic health records, and laboratory information management systems. AMSG was a wholly-owned subsidiary of Rennova that was formed on May 4, 2017 and HTS was a wholly-owned subsidiary of Rennova that was formed on June 22, 2011.
AMSG’s financial results include the assets and operations of CollabRx, Inc. and Genomas, Inc. Genomas, Inc. operated a diagnostics lab until December 31, 2019 and was focused solely on the technology and platform to interpret diagnostics outcomes and translate these outcomes into easily usable information. HTS’s financial results include the assets and operations of two other strategic businesses owned by Rennova: ClinLab, Inc.; and Medical Mime, Inc. HTS’s results do not include Platinum Financial Solutions, LLC, which was left with Rennova. After the separation, Rennova retained full ownership of its remaining businesses.
On June 25, 2021, Rennova sold all the shares of stock of its subsidiaries, HTS and AMSG, to InnovaQor in a transaction that has been accounted for as a reverse acquisition with the Group being the accounting acquirer.
In consideration for the shares of HTS and AMSG (HTS Group) and the elimination of inter-company debt between Rennova and HTS and AMSG, InnovaQor issued to Rennova 14,000 shares of its Series B-1 Convertible Redeemable Preferred Stock (the “Series B-1 Preferred Stock”). The number of shares of Series B-1 Preferred Stock was subject to a post-closing adjustment which resulted in an additional 950 shares of Series B-1 Preferred Stock due Rennova, which were issued in September 2021. Each share of Series B-1 Preferred Stock has a stated value of $1,000 and is convertible into that number of shares of InnovaQor’s common stock equal to the product of the stated value divided by 90% of the average closing price of InnovaQor’s common stock during the 10 trading days immediately prior to the conversion date. Conversion of the Series B-1 Preferred Stock, however, is subject to the limitation that no conversion can be made to the extent the holder’s beneficial interest (as defined pursuant to the terms of the Series B-1 Preferred Stock) in the common stock of InnovaQor would exceed 4.99%. The fair market value of the 14,950 shares was $9,086,396, as described below. The shares of Series B-1 Preferred Stock may be redeemed by InnovaQor upon payment of the stated value of the shares plus any declared and unpaid dividends. Because these shares are convertible, at the option of the holder, into a variable number of common shares based solely on a fixed dollar amount (stated value) known at issuance of the shares, they have been recorded as a long-term liability at the date of issuance in accordance with ASC 480, Distinguishing Liabilities from Equity.
On June 9, 2021, InnovaQor issued 1,000 shares of Series A-1 Supermajority Voting Preferred Stock (the “Series A-1 Preferred Stock”) to the then CEO of the Company, Mr. Gerard Dab, in exchange for $300,000 owed to Mr. Dab. The Series A-1 Preferred Stock has the right to the number of votes equal to 51% of the votes entitled to be cast at a meeting or to vote by written consent, meaning the owner of the Series A-1 Preferred Stock has voting control of the Company. Mr. Dab was a party to an agreement whereby he committed to transfer the Series A-1 Preferred Stock to Epizon Limited (“Epizon”), a Nassau, Bahamas, based management consulting company. Seamus Lagan, the Chief Executive Officer of Rennova, the company we ultimately completed a transaction with, is also the managing director of Epizon. The conditions of the Epizon agreement to which Mr. Dab was a party were met and the transfer of shares of Series A-1 Preferred Stock to Epizon was completed. The terms of the agreement between Mr. Dab and Epizon had certain conditions including a condition that if within 120 days after a transaction was completed by VisualMED, there were not any dispute or efforts to unwind the transaction, then Mr. Dab would deliver the shares of Series A-1 Preferred Stock owned by him to Epizon. Epizon, as the owner of the Series A-1 Preferred Stock, will be able to exercise control over all matters submitted for stockholder approval.
| F-26 |
InnovaQor issued 200 shares of Series C-1 Convertible Redeemable Preferred Stock (the “Series C-1 Preferred Stock”) to Mr. Dab in exchange for $200,000 owed to him. The shares had a fair market value of $122,000 at the date of issuance, as described below. Because these shares are convertible, at the option of the holder, into a variable number of common shares based solely on a fixed dollar amount (stated value) known at issuance of the shares, they have been recorded as a long-term liability at the date of issuance in accordance with ASC 480, Distinguishing Liabilities from Equity.
The fair market value of all of the above shares of Series B-1 and Series C-1 Preferred Stock is based on the Option Price Method (the “OPM”). The OPM treats common and preferred interests as call options on the equity value of the subject company, with exercise prices based on the liquidation preference of the preferred interests and participation thresholds for subordinated classes. The Black-Scholes model was used to price the call options. The assumptions used were: risk free rate of 0.84%, volatility of 250.0%, and exit period of 5 years. Lastly, a discount rate of 35% was applied due to the lack of marketability of the InnovaQor preferred stock and the underlying liquidity of InnovaQor’s common stock.
Additionally, Mr. Dab returned 14,465,259 shares of Common Stock in InnovaQor for cancellation.
The goal of the Company is to develop and deliver a technology-based communication platform to a broad range of healthcare professionals and businesses using a subscription revenue model with added value bolt on services.
InnovaQor has six wholly-owned subsidiaries that provide medical support services primarily to clinical laboratories, corporate operations, rural hospitals, physician practices and behavioral health/substance abuse centers.
Health Technology Solutions, Inc. (“HTS”): HTS provides information technology and software solutions to our subsidiaries and outside medical service providers. HTS provides vCIO, IT managed services and data analytics dashboards to our subsidiaries and outside medical service providers. HTS operates from the corporate offices in West Palm Beach, Florida.
Medical Mime, Inc. (“Mime”): Mime was formed on May 9, 2014. It specializes in electronic health records (EHR) software and subscription services for the behavioral health and rehabilitation market segments. It currently serves nine behavioral health/substance abuse facilities.
ClinLab, Inc. (“ClinLab”): ClinLab develops and markets laboratory information management systems to mid-size clinical laboratories. It currently services 12 clinical laboratories across the country.
AMSG owns CollabRx, Inc. (“CollabRx”) and Genomas, Inc. (“Genomas”), each of which is an inactive operation.
Genomas operated a diagnostics lab until December 31, 2019 and was focused solely on the pharmacogenomics technology and platform, MedTuning, to interpret diagnostics outcomes and translate these outcomes into easily usable information to indicate the effectiveness of medications for a patient. This solution would require minimum effort to be back in operation. CollabRx owns a technology platform and database for interpreting diagnostics outcomes from cancer patients that could match the result to known treatments and or clinical trials. This solution has been dormant for a number of years and to be viable in the marketplace will require updates to the technology and the database.
| F-27 |
Each of the subsidiaries is wholly owned by the Company and complements each other, allowing for cross selling of products and services. The Company believes the current solutions will become an added value option to a technology-based communication platform to a broad range of healthcare professionals and businesses using a subscription revenue model with added value bolt on services the Company plans to develop.
Existing products offered by the Company’s subsidiaries are as follows:
“M2Select” is a custom built, cloud based, electronic health record which meets the needs of substance abuse treatment and behavioral health providers. M2Select’s specialized clinical workflow provides intuitive prompts for symptoms and enables you to quickly select problems and create master treatment plans with goals, objectives, and interventions. M2Select provides best-in-class patient lifecycle management for Behavioral Health/Substance Abuse (BH/SA) treatment centers. From pre-admission to billing and aftercare, M2Select is an electronic health record and patient management software that seamlessly integrates into the natural workflow of day-to-day operations.
“M2Pro” is a custom built, cloud based, electronic health record for ambulatory physician practices that meets meaningful use stage 2 and no further. Its unique dictation services further automate the workflow process for physicians allowing them to focus on their continuum of patient care.
“ClinLab” is a turnkey client/server lab information system for mid-range laboratories. ClinLab supports interfaces to all major reference labs and the ClinLab team can provide an interface to any system with that capability. ClinLab also features an optional EHR package which enables interfacing with the most popular EHR systems allowing lab test results to integrate seamlessly into a provider’s EHR for an improved patient record and to fulfill the federal government requirements.
“Qira” is our healthcare business analytics service powered by PowerBI. It is a culmination of healthcare financial and revenue cycle management plus clinical operations oversight needs. It aggregates data from multiple healthcare systems to produce a single source business intelligence tool with executive level daily briefing to deep dive operational management of claims and operational efficiencies. There are many other analytical services available that customize solutions but none that have a proven template for success. Our competitive advantage comes from having created these tools to identify the deficiencies in the real world for the former parent Rennova from its former national laboratory operations to its more recent rural hospitals.
“vCIO Services”. Based on the skills and experience inherent within InnovaQor and resulting from work undertaken on behalf of the former parent, Rennova, InnovaQor offers a range of CIO services centered on our ability to link IT systems to business objectives combined with our knowledge of technology trends likely to impact our sector. The CIO services would include (but not be limited to):
| ● | Program and Project Management | |
| ● | Vendor Management | |
| ● | Business Continuity and Disaster Recovery | |
| ● | Security Services | |
| ● | Network Infrastructure Management | |
| ● | Helpdesk Provision |
| F-28 |
“MedTuning” utilizes proprietary biomarkers, treatment algorithms, and a web-based interactive physician portal delivery system to provide clinical decision support for physicians and personalized drug treatment for patients. Products are DNA-guided to improve the therapeutic benefit of widely used prescription drugs while also reducing the risk of significant side effects for patients.
Medical Informatics: Our technology platform, proprietary algorithms and physician interface portal can be extended to a wide range of drug categories.
Research and Development: Technology platform applicable to numerous disease states; current pipeline in mental health, pain management, cardiovascular and diabetes.
“Advantage” is a proprietary HIPAA compliant software developed to eliminate the need for paper requisitions by providing an easy to use and efficient web-based system that lets customers securely place lab orders, track samples and view test reports in real time from any web-enabled laptop, notepad or smart phone.
In the coming year we plan to develop, acquire or license and offer a telehealth solution through corporate partnerships in the emerging health technology sector.
Note 2 - Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The acquisition of an operating company by a non-operating public shell corporation typically results in the owners and management of the operating company having actual or effective voting and operating control of the combined company. The Securities and Exchange Commission staff considers a public shell reverse acquisition to be a capital transaction in substance, rather than a business combination. That is, the transaction is a reverse recapitalization, equivalent to the issuance of stock by the operating company for the net monetary assets of the shell corporation accompanied by a recapitalization. The accounting is similar to that resulting from a reverse acquisition, except that no goodwill or other intangible assets are recorded.
The condensed consolidated financial statements include the accounts of only the HTS Group (the accounting acquirer) prior to June 25, 2021 and InnovaQor and the Group since the date of acquisition on June 25, 2021, with the transaction being accounted for as a recapitalization of the Group on June 25, 2021. The condensed consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and require management to make certain judgments, estimates, and assumptions. These may affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements. They also may affect the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates upon subsequent resolution of identified matters.
The accompanying condensed consolidated financial statements as of and for the six months ended June 30, 2022 and 2021, have been derived from unaudited financial information. Intercompany accounts and transactions have been eliminated. The accompanying unaudited interim condensed consolidated financial statements have been prepared on the same basis as the annual audited financial statements and in accordance with U.S. GAAP, for interim financial information and the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim financial statements. In the opinion of management, such unaudited information includes all adjustments (consisting only of normal recurring accruals) necessary for a fair presentation of this interim information.
| F-29 |
Comprehensive Loss
During the six months ended June 30, 2022 and 2021, comprehensive loss was equal to the net loss amounts presented in the accompanying condensed consolidated statements of operations.
Going Concern
Under Accounting Standards Update (“ASU”) 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40) Accounting Standards Codification (“ASC 205-40”), the Company has the responsibility to evaluate whether conditions and/or events raise substantial doubt about its ability to meet its future financial obligations as they become due within one year after the date that the financial statements are issued. As required by ASC 205-40, this evaluation shall initially not take into consideration the potential mitigating effects of plans that have not been fully implemented as of the date the financial statements are issued. Management has assessed the Company’s ability to continue as a going concern in accordance with the requirement of ASC 205-40.
The accompanying consolidated financial statements have been prepared in accordance with U.S. GAAP and the rules and regulations of the SEC. The consolidated financial statements have been prepared using U.S. GAAP applicable to a going concern that contemplates the realization of assets and liquidation of liabilities in the normal course of business. The Company has accumulated significant losses and has negative cash flows from operations and, at June 30, 2022, had a working capital deficit and accumulated deficit of $3.5 million and $18.6 million, respectively. In addition, the Company’s cash position is critically deficient and critical payments are not being made in the ordinary course of business, all of which raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans with respect to alleviating the adverse financial conditions that caused management to express substantial doubt about the Company’s ability to continue as a going concern are as follows:
The Company will incur substantial costs in connection with the acquisition of the Group from Rennova, which may include accounting, tax, legal and other professional services costs, recruiting and relocation costs associated with hiring key senior management personnel who are new to the Company, tax costs and costs to separate information systems, among other costs. The cost of performing such functions is anticipated to be higher than the amounts reflected in the Company’s historical financial statements, which would cause its future losses to increase. Accordingly, the Company will continue to focus on reducing its operating costs and increasing revenues.
There can be no assurance that the Company will be able to achieve its business plan, raise any additional capital or secure the additional financing necessary to implement its current operating plan. The ability of the Company to continue as a going concern is dependent upon its ability to increase its revenues and eventually achieve profitable operations. The accompanying condensed consolidated financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant areas of estimation include estimating the fair value of intangible assets acquired, the impairment of assets, accrued and contingent liabilities, and future income tax obligations (benefits), among other items. Actual results could differ from those estimates and would impact future results of operations and cash flows.
| F-30 |
Cash and Cash Equivalents
The Company considers all highly liquid temporary cash investments with an original maturity of three months or less to be cash equivalents.
Allowance for Doubtful Accounts Policy
Accounts receivable are reported at realizable value, net of allowances for doubtful accounts, which are estimated and recorded in the period that the Company deems the receivable to be uncollectable. The Company has a standardized approach to estimate and review the collectability of its receivables based on a number of factors, including the period they have been outstanding. Historical collection is an integral part of the estimation process related to the allowance for doubtful accounts. In addition, the Company regularly assesses the state of its billing operations in order to identify issues that may impact the collectability of these receivables or reserve estimates. Receivables deemed to be uncollectible are charged against the allowance for doubtful accounts at the time such receivables are written-off. Recoveries of receivables previously written-off are recorded as credits to the allowance for doubtful accounts. Revisions to the allowances for doubtful accounts estimates are recorded as an adjustment to the provision for bad debts.
Revenue Recognition
We recognize revenue in accordance with Accounting Standards Update (“ASU”) 2014-09, “Revenue from Contracts with Customers (Topic 606),” including subsequently issued updates. This series of comprehensive guidance has replaced all existing revenue recognition guidance. There is a five-step approach outlined in the standard. In determining revenue, we first identify the contract according to the scope of ASU Topic 606 with the following criteria:
| ● | Identify the contract(s) with a customer. | |
| ● | Identify the performance obligations in the contract. | |
| ● | Determine the transaction price. | |
| ● | Allocate the transaction price to the performance obligations in the contract. | |
| ● | Recognize revenue when or as you satisfy a performance obligation. |
Revenue is recognized when control of the promised services is transferred to the Company’s customers in an amount that reflects the consideration the Company is expected to be entitled to in exchange for those services. As the Company completes its performance obligations which are identified in Note 10 below, it has an unconditional right to consideration as outlined in the Company’s contracts. Generally, the Company’s accounts receivable are expected to be collected in 30 days in accordance with the underlying payment terms. For many of the Company’s services, the Company typically has one performance obligation; however, it also provides the customer with an option to acquire additional services. The Company typically provides a menu of offerings from which the customer may choose to purchase. The price of each service is generally based upon an agreed hourly rate.
Impairment or Disposal of Long-Lived Assets
The Company accounts for the impairment or disposal of long-lived assets according to the Financial Accounting Standards Board’s (“FASB”) ASC 360, “Property, Plant and Equipment.” Long-lived assets are reviewed when facts and circumstances indicate that the carrying value of the asset may not be recoverable. When necessary, impaired assets are written down to estimated fair value based on the best information available. Estimated fair value is generally based on either appraised value or measured by discounting estimated future cash flows. Considerable management judgment is necessary to estimate discounted future cash flows. Accordingly, actual results could vary significantly from such estimates. As of June 30, 2022 and 2021, all of the Company’s fixed assets were fully depreciated and, therefore, the carrying value of fixed assets represented fair value. Fixed assets are depreciated over lives ranging from three to seven years.
| F-31 |
Fair Value of Financial Instruments
In accordance with ASC 820, “Fair Value Measurements and Disclosures,” the Company applies fair value accounting for all financial assets and liabilities and non-financial assets and liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis. Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities that are required to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and the market-based risk measurements or assumptions that market participants would use in pricing the asset or liability, such as risks inherent in valuation techniques, transfer restrictions and credit risk. Fair value is estimated by applying the following hierarchy, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement:
| ● | Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. | |
| ● | Level 2 applies to assets or liabilities for which there are inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly, such as quoted prices for similar assets or liabilities in active markets; or quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets). | |
| ● | Level 3 applies to assets or liabilities for which fair value is derived from valuation techniques in which one or more significant inputs are unobservable, including the Company’s own assumptions. |
The estimated fair value of financial instruments is determined by the Company using available market information and valuation methodologies considered to be appropriate. At June 30, 2022 and December 31, 2021, the carrying value of the Company’s accounts receivable, accounts payable, accrued expenses and notes payable, approximate their fair values due to their short-term nature. For the three and six months ended June 30, 2022 and 2021, there were no realized and unrealized gains on instruments valued using fair value evaluation methods.
Income Taxes
The entities within the Group were included in the consolidated income tax returns of its Parent for the years ended December 31, 2020 and prior. A determination has been made by Parent’s management not to allocate any of the deferred tax assets or liabilities to the Group as of December 31, 2020 and prior. Accordingly, the Group had not provided for income taxes in the combined financial statements. The Company since June 25, 2021 uses the liability method of accounting for income taxes. Under the liability method, future tax liabilities and assets are recognized for the estimated future tax consequences attributable to differences between the amounts reported in the financial statement carrying amounts of assets and liabilities and their respective tax bases. Future tax assets and liabilities are measured using enacted or substantially enacted income tax rates expected to apply when the asset is realized or the liability settled. The effect of a change in income tax rates on future income tax liabilities and assets is recognized in income in the period that the change occurs. Future income tax assets are recognized to the extent that they are considered more likely than not to be realized. When projected future taxable income is insufficient to provide for the realization of deferred tax assets, the Company will recognize a valuation allowance.
In accordance with U.S. GAAP, the Company has determined whether a tax position of the Company is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The tax benefit to be recognized is measured as the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement. Derecognition of a tax benefit previously recognized could result in the Company recording a tax liability that would reduce net assets. The Company has determined that it has not incurred any liability for tax benefits as of June 30, 2022 and 2021. State income taxes will also be due on any income generated in the future.
| F-32 |
Convertible Preferred Stock
The Company classifies its Series B-1 and Series C-1 Convertible Preferred Stock as liabilities in accordance with ASC 480 Distinguishing Liabilities from Equity since the preferred stock is convertible, at the option of the holder, into a variable number of shares based solely on a fixed dollar amount (stated value) known at issuance of the preferred stock.
Basic and Diluted Net Income (Loss) Per Share
The Company computes net income (loss) per share in accordance with ASC Topic 260, “Earnings per Share” which requires presentation of both basic and diluted earnings per share (EPS) on the face of the income statement. Basic EPS is computed by dividing net income (loss) available to common shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period. Diluted EPS gives effect to all dilutive potential common shares outstanding during the period including stock options, using the treasury stock method, and convertible preferred stock, using the if-converted method. As of June 30, 2022 and 2021, there were approximately 2,845,200,000 and 827,562,500 common stock equivalents, respectively, which where antidilutive due to the Company’s losses.
Note 3 – Acquisition
The Company acquired all the common stock of the HTS Group from Rennova on June 25, 2021 in exchange for Preferred Series A-1, B-1 and C stock with a fair market value of $9,195,692. This acquisition has been accounted for as a reverse acquisition with the HTS Group being the accounting acquiror with the excess fair value of the purchase price over net asset fair value acquired treated as a reduction of additional paid in capital on the date of acquisition.
A summary of that purchase price is as follows:
Schedule of Purchase Price
| Fair Value of Preferred Series A-1 Stock | $ | 100 | ||
| Fair Value of Preferred Series B-1 Stock | $ | 9,086,396 | ||
| Fair Value of Preferred Series C-1 Stock | $ | 122,000 | ||
| Other | $ | (12,804 | ) | |
| Total | $ | 9,195,692 |
On the date of acquisition, InnovaQor had no assets and total liabilities of $500,035, of which $500,000 was converted to equity in connection with the acquisition.
Unaudited Statements of Operations for the three and six months ended June 30, 2021, as if the acquisition had taken place on January 1, 2021, would show an additional loss of approximately $500,000 due to additional consulting expense.
| F-33 |
Note 4 – Accounts Receivable
Accounts receivable at June 30, 2022 and December 31, 2021 consisted of the following:
Schedule of Accounts Receivable
| June 30, | December 31, | |||||||
| 2022 | 2021 | |||||||
| Accounts receivable (including related party of $12,327 and $21,852 at June 30, 2022 and December 31, 2021, respectively) | $ | 52,837 | $ | 32,800 | ||||
| Less: | ||||||||
| Allowance for discounts | — | — | ||||||
| Accounts receivable, net | $ | 52,837 | $ | 32,800 | ||||
For the three months ended June 30, 2022 and 2021, bad debt expense (recovery), was $0 and $4,279, respectively.
For the six months ended June 30, 2022 and 2021, bad debt expense (recovery), was $0 and $4,279, respectively.
Note 5 – Property and Equipment
Property and equipment at June 30, 2022 and December 31, 2021 consisted of the following:
Summary of Property and Equipment
| June 30, | December 31, | |||||||
| 2022 | 2021 | |||||||
| Software | $ | 1,435,875 | $ | 1,435,875 | ||||
| Furniture | 8,227 | 8,227 | ||||||
| Office equipment | 30,931 | 30,931 | ||||||
| Computer equipment | 324,131 | 324,131 | ||||||
| 1,799,164 | 1,799,164 | |||||||
| Less accumulated depreciation | (1,799,164 | ) | (1,799,164 | ) | ||||
| Property and equipment, net | $ | — | $ | — | ||||
Depreciation expense on property and equipment was $0 and $237 for the three months ended June 30, 2022 and 2021, respectively, and $0 and $711 for the six months ended June 30, 2022 and 2021, respectively. Management periodically reviews the valuation of long-lived assets, including property and equipment, for potential impairment.
| F-34 |
Note 6 – Accrued Expenses
Accrued expenses at June 30, 2022 and December 31, 2021 consisted of the following:
Schedule of Accrued Expenses
| June 30, | December 31, | |||||||
| 2022 | 2021 | |||||||
| Accrued payroll and related liabilities | $ | 1,211,212 | $ | 1,263,539 | ||||
| Accrued legal | 37,996 | 37,997 | ||||||
| Accrued interest | 23,527 | 17,494 | ||||||
| Deferred revenue and customer deposits | 44,544 | 24,471 | ||||||
| Other accrued expenses | 120,591 | 103,221 | ||||||
| Accrued expenses | $ | 1,437,870 | $ | 1,446,722 | ||||
Accrued payroll and related liabilities at June 30, 2022 and December 31, 2021 included approximately $1.0 million and $1.1 million, respectively, of accrued past due payroll taxes, related penalties and interest.
Note 7 – Notes Payable
The carrying amount of notes payable as of June 30, 2022 and December 31, 2021 was as follows:
Schedule of Notes Payable
| June 30, | December 31, | |||||||
| 2022 | 2021 | |||||||
| Note payable with the Department of Economic and Community Development in the original amount of $147,372 due in monthly payments of principal and interest totaling $2,132 beginning January 1, 2017 with a final payment due on October 1, 2022. Non-interest bearing. Payments were not made in 2022 or 2021. | $ | 134,153 | $ | 134,153 | ||||
| Loan from Company’s CEO, due September 6, 2022. Original issue discount of $8,410; 25 shares of Series C-1 Preferred Stock issued in connection with the loan; unamortized debt discount of $8,676 at June 30, 2022 | 89,799 | - | ||||||
| Paycheck Protection Program Loans (PPP Loans). The PPP Loans and accrued interest are forgivable as long as the Company uses the loan proceeds for eligible purposes, including payroll, benefits, rent and utilities, and maintains its payroll levels. The amount of loan forgiveness will be reduced if the Company terminates employees or reduces salaries. No collateral or guarantees were provided in connection with the PPP Loans. The unforgiven portion of the PPP Loans are payable over two years at an interest rate of 1.0% per annum, with a deferral of payments for the first sixteen months. Beginning sixteen months from the dates of issuance, the Company is required (if not forgiven) to make monthly payments of principal and interest to the lenders. The Company intends to use all of the proceeds for purposes consistent with the PPP. While the Company currently believes that its use of the loan proceeds will meet the conditions for forgiveness of the loans, it cannot assure you that it will not take actions that could cause the Company to be ineligible for forgiveness of the loans, in whole or in part. | 60,401 | 60,401 | ||||||
| 284,353 | 194,554 | |||||||
| Less current portion | 223,952 | 134,153 | ||||||
| Notes payable, long term, net of current portion | $ | 60,401 | $ | 60,401 | ||||
| F-35 |
Note 8 – Loans from Parent and Other Related Party Transactions
To fund the Company’s operations for the three and six months ended June 30, 2022 and 2021, the former Parent advanced funds and paid expenses of InnovaQor and the Group in the amount of $89,518 and $428,943, respectively. The amount as of June 30, 2022 is included in Due to Former Parent in the accompanying Condensed Consolidated Balance Sheet.
During the three and six months ended June 30, 2022, Ms. Hollis, the Chief Executive Officer of the Company, loaned the Company $90,100. The Company entered into a promissory note in the amount of $98,510, representing a 10% original issue discount. The loan is due on September 6, 2022; provided that, 50% is due if the Company receives $500,000 of financing and 100% is due if the Company receives $1,000,000 of financing. In addition, the Company issued Ms. Hollis 25 shares of Series C-1 Preferred Stock on March 31, 2022 in connection with the loan. These shares of Series C-1 Preferred Stock were valued at $15,250 using the Option Price Method and the same assumptions as used to value the prior issuance of Series C-1 Preferred Stock.
See also Note 14.
The above amounts are not indicative of what third parties would have agreed to.
Related Parties Revenue
Included in net revenues for the three months ended June 30, 2022 and 2021 is $47,788 and $62,316, respectively, of intercompany revenue with Rennova (the former parent). Included in net revenues for the six months ended June 30, 2022 and 2021 is $102,267 and $124,632, respectively, of intercompany revenue with Rennova (the former parent).
The Group has incurred certain costs that have been allocated from Rennova. Included in the Condensed Consolidated Statements of Operations are the following allocated costs:
Schedule of Allocated Costs
| Three Months Ended June 30, | Three Months Ended June 30, | |||||||
| 2022 | 2021 | |||||||
| Health insurance | $ | — | $ | 4,011 | ||||
| Rent and utilities | 33,411 | 20,135 | ||||||
| Total allocated costs | $ | 33,411 | $ | 24,146 | ||||
Six Months Ended June 30, | Six Months Ended June 30, | |||||||
| 2022 | 2021 | |||||||
| Health insurance | $ | — | $ | 11,130 | ||||
| Rent and utilities | 62,364 | 40,136 | ||||||
| Total allocated costs | $ | 62,364 | $ | 51,266 | ||||
| F-36 |
Note 9 – Preferred Stock and Stockholders’ Deficit
Common Stock
The Company has authorized 325,000,000 shares of $0.0001 par value Common Stock of which 234,953,286 were issued and outstanding as of June 30, 2022 and December 31, 2021. These shares have one vote per share.
Preferred Stock Series A-1
The Company has authorized 1,000 shares of $0.0001 par value (stated value $10) Series A-1 Supermajority Voting Preferred Stock of which 1,000 were issued and outstanding as of June 30, 2022 and December 31, 2021. So long as one share of Series A-1 Preferred Stock is outstanding, the outstanding shares of the Series A-1 Preferred Stock shall have the number of votes, in the aggregate, equal to 51% of all votes entitled to be voted at any stockholder meeting. These shares have no rights to receive dividends and liquidation rights are equal to the stated value per share.
Preferred Stock Series B-1
The Company has authorized 25,000 shares of $0.0001 par value (stated value $1,000) Series B-1 Convertible Redeemable Preferred Stock of which 14,950 were issued and outstanding as of June 30, 2022 and December 31, 2021. These shares have no voting rights, dividends on these shares shall accrue at the rate of 5% of the stated value per share and liquidation rights are equal to the stated value per share. These shares are convertible into the Company’s Common Stock based on the stated value at a conversion price equal to 90% of the average closing price of the Common Stock on the 10 Trading Days immediately prior to the Conversion Date but in any event no less than the par value of the Common Stock. The Series B-1 Preferred Stock was not convertible prior to the first anniversary of its issuance except with the consent of the holders of a majority of the then outstanding shares, if any, of the Series A-1 Preferred Stock. No conversion can be made to the extent the holder’s beneficial interest (as defined pursuant to the terms of the Series B-1 Preferred Stock) in the common stock of InnovaQor would exceed 4.99%. These shares are redeemable at the option of the Company at their stated value plus declared and unpaid dividends. Because these shares are convertible, at the option of the holder, into a variable number of common shares based solely on a fixed dollar amount (stated value) known at issuance of the shares, they have been recorded as a long-term liability at the date of issuance in accordance with ASC 480, Distinguishing Liabilities from Equity.
| F-37 |
Preferred Stock Series C-1
The Company has authorized 2,000 shares of $0.0001 par value (stated value $1,000) Series C-1 Convertible Redeemable Preferred Stock of which 225 and 200 were issued and outstanding as of June 30, 2022 and December 31, 2021, respectively. These shares have no voting rights, dividends on these shares shall accrue at the rate of 10% of the stated value per share and liquidation rights are equal to the stated value per share. These shares are convertible into the Company’s Common Stock based on the stated value at a conversion price equal to 90% of the average closing price of the Common Stock on the 10 Trading Days immediately prior to the Conversion Date but in any event no less than the par value of the Common Stock. The Series C-1 Preferred Stock was not convertible prior to the first anniversary of its original issuance except with the consent of the holders of a majority of the then outstanding shares, if any, of the Series A-1 Preferred Stock. No conversion can be made to the extent the holder’s beneficial interest (as defined pursuant to the terms of the Series C-1 Preferred Stock) in the common stock of InnovaQor would exceed 4.99%. These shares are redeemable at the option of the Company at their stated value plus declared and unpaid dividends. Because these shares are convertible, at the option of the holder, into a variable number of common shares based solely on a fixed dollar amount (stated value) known at issuance of the shares, they have been recorded as a long-term liability at the date of issuance in accordance with ASC 480, Distinguishing Liabilities from Equity.
Note 10 – Revenue
The Company had net revenue for the three and six months ended June 30, 2022 and 2021 as follows:
Schedule of Net Revenue
| Three Months | Three Months | |||||||
| Ended June 30, | Ended June 30, | |||||||
| 2022 | 2021 | |||||||
| Dashboards | $ | 10,478 | $ | 14,076 | ||||
| IT Managed Services | 22,620 | 25,740 | ||||||
| Software and Interfaces | 5,580 | - | ||||||
| Support and Maintenance | 26,895 | 24,363 | ||||||
| vCIO Services | 13,240 | 22,500 | ||||||
| Software Licenses Fees | 15,247 | 17,997 | ||||||
| Other | 451 | - | ||||||
| Total Net Revenue | $ | 94,511 | $ | 104,676 | ||||
| Six Months | Six Months | |||||||
| Ended June 30, | Ended June 30, | |||||||
| 2022 | 2021 | |||||||
| Dashboards | $ | 24,102 | $ | - | ||||
| IT Managed Services | 48,360 | 46,397 | ||||||
| Software and Interfaces | 5,580 | 8,840 | ||||||
| Support and Maintenance | 52,736 | 118,580 | ||||||
| vCIO Services | 27,431 | - | ||||||
| Software Licenses Fees | 30,744 | 43,124 | ||||||
| Other | 2,374 | - | ||||||
| Total Net Revenue | $ | 191,327 | $ | 216,941 | ||||
Generally, work is billed monthly by the hour at agreed upon hourly rates for all of the above revenue streams.
For all of the Company’s services, the Company typically has one performance obligation; however, it also provides the customer with an option to acquire additional services. The Company typically provides a menu of offerings from which the customer may choose to purchase. The price of each service is separate and distinct and provides a separate and distinct value to the customer. Pricing is generally consistent for each service irrespective of the other services or quantities requested by the customer.
When the Company receives consideration from a customer prior to transferring services to the customer under the terms of the contract, it records deferred revenues on the Company’s consolidated balance sheet, which represents a contract liability.
The Company has an internal sales force compensation program where remuneration is based solely on the revenues recognized in the period and does not represent an incremental cost to the Company which provides a future benefit expected to be longer than one year and would meet the criteria to be capitalized and presented as a contract asset on the Company’s consolidated balance sheet.
| F-38 |
Note 11 – Commitments and Contingencies
Consulting Agreement – the Company entered into a consulting agreement effective June 1, 2021, with Dial M Productions LLC, a company owned by Mr. Dab, the Company’s former CEO, for a period of one year to provide assistance in developing the Company’s business including communications with existing shareholders and the general public. The agreement provided that the Company would pay $3,500 a month and an additional $60,000 upon the earlier of receipt of funding from an outside source or within 90 days. These amounts have not been paid. On June 1, 2022, the agreement was extended for another year. The Company continues to owe the amounts provided for in the original agreement and the new agreement increases the monthly fee to $4,500.
Concentration of Credit Risk - Credit risk with respect to accounts receivable is generally low due to the nature of the customers comprising the customer base and the significant related party component. The Company does not require collateral or other security to support customer receivables. However, the Company continually monitors and evaluates its client acceptance and collection procedures to minimize potential credit risks associated with its accounts receivable and establishes an allowance for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is not material to the condensed consolidated financial statements.
The Company maintains its cash balances in high-credit-quality financial institutions. The Company’s cash balances may, at times, exceed the deposit insurance limits provided by the Federal Deposit Insurance Corp.
Guarantees
Certain subsidiaries of the Company have guaranteed debt obligations of their former Parent. As part of the transaction with the Company, the former Parent received a release of guarantees from certain institutional lenders and has been working to settle other debt obligations where certain subsidiaries of the Company remain a guarantor. The Company believes that any risk associated with previous guarantees is now minimal and immaterial.
Legal Matters
From time to time, the Company may be involved in a variety of claims, lawsuits, investigations and proceedings related to contractual disputes, employment matters, regulatory and compliance matters, intellectual property rights and other litigation arising in the ordinary course of business. The Company operates in a highly regulated industry which may inherently lend itself to legal matters. Management is aware that litigation has associated costs and that results of adverse litigation verdicts could have a material effect on the Company’s condensed consolidated financial position or results of operations. Management, in consultation with legal counsel, has addressed known assertions and predicted unasserted claims below.
P2P Staffing Corp. received a judgment against HTS during 2018 in the amount of $58,784 plus accrued interest and court costs for amounts owed. As of each of June 30, 2022 and December 31, 2021, $10,464 was outstanding and owed for this judgment and included in accounts payable in the accompanying Condensed Consolidated Balance Sheets.
Two former employees of CollabRx, Inc., one of the acquired subsidiaries, filed suits in a California state court against the former Parent, Rennova, and CollabRx, Inc., in connection with amounts claimed to be owed under their respective employment agreements with CollabRx, Inc. One former employee received a judgment for approximately $253,000, which Rennova has paid in full. The other former employee received a judgment for approximately $173,000.
| F-39 |
ClinLab, Medical Mime and HTS, as well as the former Parent, Rennova and many of its subsidiaries, were defendants in a case filed in Broward County Circuit Court by TCA Global Credit Master Fund, L.P. The plaintiff alleged a breach by Medytox Solutions, Inc. of its obligations under a debenture and claimed damages in excess of $2,030,000 plus interest, costs and fees. The other entities were sued as alleged guarantors of the debenture. In May 2020, the SEC appointed a Receiver to close down the TCA Global Credit Master Fund, L.P. In September 2021, the parties entered into a settlement agreement with the Receiver to pay $500,000 as full and final settlement of all claims in this matter, which amount has now been paid as of April 2022.
CTI Consulting LLC filed suit against HTS in September 2021, claiming approximately $45,000 as owed for services provided. The Company has agreed to pay $5,000 per month until the obligation is satisfied.
Note 12 – Supplemental Disclosure of Cash Flow Information
Schedule of Supplemental Disclosure of Cash Flow Information
| Six
Months Ended June 30, | ||||||||
| 2022 | 2021 | |||||||
| Cash paid for interest | $ | 5,937 | $ | 8,737 | ||||
| Cash paid for income taxes | $ | — | $ | — | ||||
| Series C-1 Preferred Stock issued in connection with debt | $ | 15,250 | $ | — | ||||
| Series A-1, B-1 & C-1 Preferred Stock issued for transaction | $ | — | $ | 9,208,396 | ||||
Note 13 – Recent Accounting Pronouncements
All recent accounting standards issued by the FASB, including its Emerging Issues Task Force, the American Institute of Certified Public Accountants, and the SEC did not or are not believed by management to have a material impact on the Company’s consolidated financial statements.
Note 14 – Subsequent Events
As of July 1, 2022, the outstanding loan from Rennova to the Company totaled $803,416. The Company signed a promissory note, dated July 1, 2022, in favor of Rennova that provided that the Company will pay Rennova $883,757 on December 31, 2022. That amount represented a 10% original issue discount above the loan amount outstanding on July 1, 2022.
From July 29, 2022 through September 1, 2022, Alcimede Limited, of which Seamus Lagan is the director, has loaned the Company an aggregate of $32,500. These loans are due on demand. Upon any repayment, the Company will also owe an amount equal to 10% of the amount repaid.
Sharon Hollis, the Chief Executive Officer of the Company, subsequent to June 30, 2022 has advanced a further $6,000 to the Company, which is repayable on demand.
| F-40 |
PART III EXHIBITS
Index to Exhibits
| Exhibit No.: | Description of Exhibit | Incorporated by Reference to: | ||
| 2. Charter and Bylaws | ||||
| 2.1 | Articles of Incorporation, as amended, of InnovaQor, Inc. | Exhibit 3.1(i) to Form 10 filed July 29, 2022 | ||
| 2.2 | Bylaws of InnovaQor, Inc. | Exhibit 3.1(ii) to Form 10 filed July 29, 2022 | ||
| 4. Subscription Agreement | ||||
| 4.1 | Subscription Agreement | Filed herewith | ||
| 6. Material contracts | ||||
| 6.1 | Consulting Agreement, dated as of May 2, 2021, between Epizon Limited and Gerard Dab* | Exhibit 10.1 to Form 10 filed July 29, 2022 | ||
| 7. Plan of acquisition, reorganization, arrangement, liquidation, or succession | ||||
| 7.1 | Acquisition Agreement, dated as of May 12, 2021, between Rennova Health, Inc. and VisualMED Clinical Solutions Corporation, as supplemented on June 23, 2021 | Exhibit 2.1 to Form 10 filed July 29, 2022 | ||
| 11. Consents | ||||
| 11.1 | Consent of Independent Registered Public Accounting Firm | Filed herewith | ||
| 11.2 | Consent of Newlan Law Firm, PLLC (see Exhibit 12.1) | Filed herewith | ||
| 12. Opinion re: Legality | ||||
| 12.1 | Opinion of Newlan Law Firm, PLLC | Filed herewith | ||
| 44 |
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this offering statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of West Palm Beach, State of Florida, on October 27, 2022.
| INNOVAQOR, INC. | ||||
| By: | /s/ Sharon Hollis | |||
| Sharon Hollis | ||||
| President and Chief Executive Officer | ||||
This Offering Statement has been signed by the following persons in the capacities and on the dates indicated.
| /s/ Sharon Hollis | October 27, 2022 | ||
| Sharon Hollis | |||
| President, Chief Executive Officer, and Director | |||
| /s/ Thomas J. Bellante | October 27, 2022 | ||
| Thomas J. Bellante | |||
| Acting Chief Financial Officer | |||
| [Principal Accounting Officer] | |||
| /s/ Justin Doherty | October 27, 2022 | ||
| Justin Doherty | |||
| Chairman and Director | |||
| /s/ Gerard Dab | October 27, 2022 | ||
| Gerard Dab | |||
| Secretary, Director |
| 45 |